Figure 9.
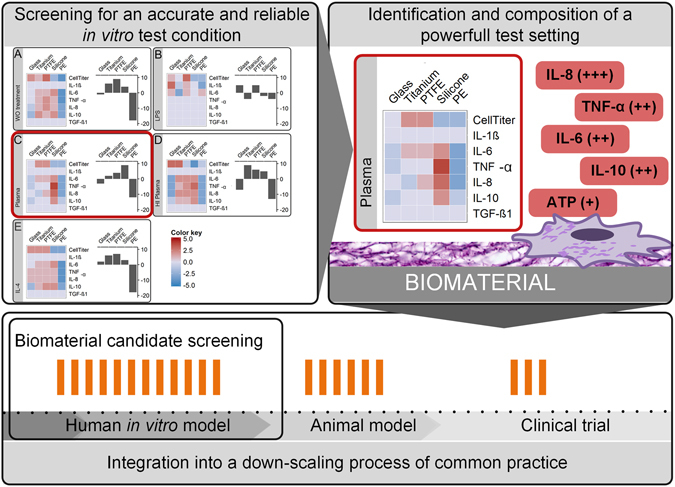
The integration of human-based in vitro test models into a down-scaling process of common practice is often neglected. One major flaw is a low correlation of in vitro tests to in vivo studies. In this study we propose a procedure to overcome this hurdle. In a large-scale in vitro screening approach, we identified an accurate and suitable condition for biomaterial testing and proved its validity by correlation to a well-described effect-the fibrotic progression - observed in vivo. On basis of this screening approach a well-defined in vitro test setting with a high predictive power was composed of (I) plasma pretreatment of biomaterials’ surface and (II) a set of highly predictive readout factors. We hope that the obtained data justifies a down-scaling of the in vitro test procedure to the identified condition and will be applied for large-scale biomaterial candidate screenings in following studies. Finally, followed by current common practice to study promising candidates in systemic animal models, and carrying the positively-proofed biomaterials in human subjects.
