Fig. 1.
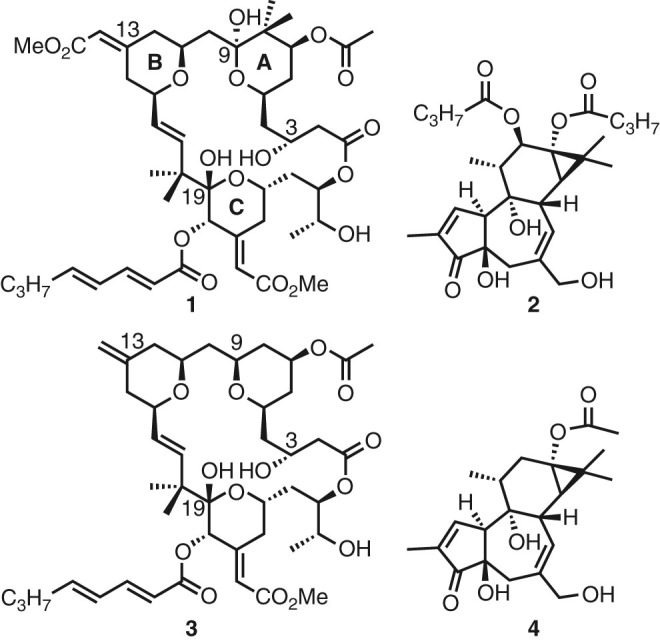
Structures of compounds simulated. Clockwise from top left: bryostatin (1), PDBu (2), prostratin (4), and a bryolog (3). Atom numberings of bryostatin are labeled, as well as bryostatin’s A, B, and C rings. Bryostatin is in the clinic for the treatment of Alzheimer’s disease9 and for HIV/AIDS eradication11. Prostratin shows PKC selectivities similar to bryostatin (albeit with lower affinities) and is a preclinical lead for HIV eradication. PDBu is similar in structure to prostratin, but unlike it is representative of the tumor-promoting phorbol diesters. Bryolog 3, synthesized by Keck and coworkers, is structurally similar to bryostatin itself but has been shown to behave quite differently in biological assays
