Figure 6.
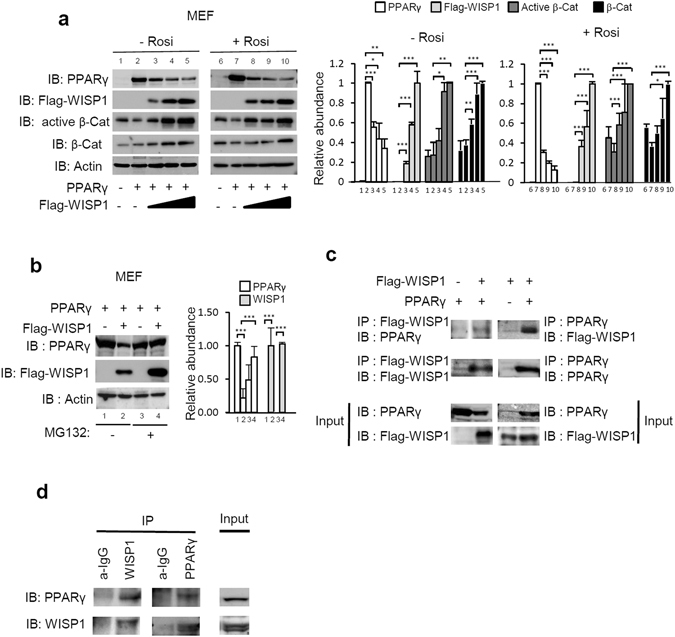
WISP1 interacts with and down-regulates PPARγ (a) MEF cells were transfected with increasing amounts of Flag-WISP1 expression vector (0.65, 1.30 and 2.60 µg) in combination with 1 µg of PPARγ expression vector. After 4 hours, cells were treated with 1 µM of rosiglitazone and harvested 24 hours later. Lysates were subjected to immunoblotting with anti-PPARγ, anti-Flag, anti-active β-catenin or anti-β-catenin antibodies. Immunoblot signals were quantified by densitometry, and normalized with β-actin. Data were expressed as means ± SEM; *p < 0.05; **p < 0.01; ***p < 0.005. Data are representative of 3 independent experiments (b) MEF cells were transfected with 2 µg of Flag-WISP1 expression vector in combination with 2 µg of PPARγ expression vector. After 24 hours, cells were treated with 10 µM MG132 for 6 hours and harvested. Lysates were subjected to immunoblotting with anti-PPARγ and anti-Flag antibodies. Immunoblot signals were quantified by densitometry, and normalized with β-actin. Data were expressed as means ± SEM; *p < 0.05; **p < 0.01; ***p < 0.005. Data are representative of 3 independent experiments (c) MEF cells were transfected with 2 µg of Flag-WISP1expression vector in combination with 2 µg of PPARγ expression vector for 24 hours. Lysates were subjected to anti-Flag or anti-PPARγ immunoprecipitation (IP) and followed by immunoblotting (IB) with anti-PPARγ or anti-Flag antibodies respectively. Protein expressions were determined by direct immunoblotting (Input). Two independent experiments were performed. (d) MEF cells lysates were subjected to anti-WISP1 or anti-PPARγ immunoprecipitation (IP) and followed by immunoblotting (IB) with anti-PPARγ or anti-WISP1 antibodies, respectively. Immunoprecipitations with IgG were used as controls. Protein expressions were determined by direct immunoblotting (Input). Two independent experiments were performed.
