Figure 7.
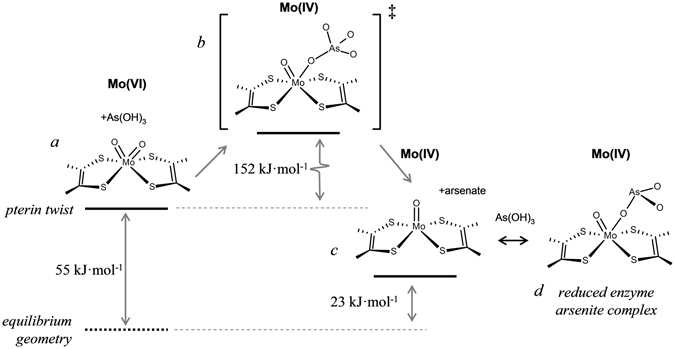
Schematic diagram of postulated reaction sequence for Aio. The initial cis-dioxo Mo(VI) site (a) is destabilized by some 55 kJ/mol from the pterin-twist induced by the protein, and reacts with arsenite in the active site pocket via a transition state (b) containing arsenic bound to molybdenum via a linking oxygen. This then breaks down to form the reduced Mo(IV) enzyme site plus the reaction product arsenate, (c). The Mo(IV) form observed with bound arsenic in the presence of excess substrate (arsenite) is also shown in (d), which we suggest to be a dead-end complex of reduced enzyme and arsenite. The oxidized active site is regenerated from (c) through intramolecular electron transfer to the other redox active centers, and reaction with solvent water (not shown) to form the cis-dioxo active site a, completing the catalytic cycle.
