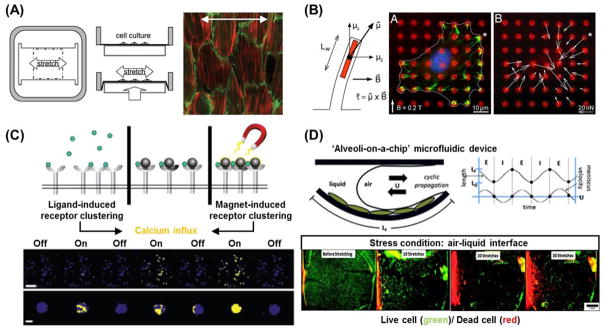
Figure 10.
Dynamic platforms for applying forces to cultured cells. (A) Cyclic uniaxial stretching device designed stretch chamber and indenter geometries. The extensions at the corners of the indenter increase the uniformity of the strain field over the indenter, resulting in a virtually uniaxial stretch. Actin stress fibers (red) orient perpendicular to the direction of cyclic stretch (white double arrow) in cultured arterial endothelial cells with normal Rho activity. Cell borders are indicated by β-catenin staining (green) [178]. (B) Magnetic post actuation device using electromagnets. Representative immunofluorescent micrograph of focal adhesions (green), microposts (red), and nucleus (blue) after force actuation. The direction and magnitude of the field are shown. The cell is outlined, and the location of the magnetic post is marked by the asterisk (*). FA proteins recruit to site of external force application [189]. (C) Nanomagnetic manipulation of receptor signal transduction. The biochemical mechanism that stimulates downstream signaling (left) involves the binding of multi-valent ligands (small green hexagons) that induce oligomerization of individual IgE/FceRI receptor complexes. Monovalent ligand-coated magnetic nanobeads (dark grey circles) bind individual IgE/Fc1RI receptor complexes without inducing receptor clustering (center). However, applying a magnetic field that magnetizes the beads and pulls them into tight clusters (right) rapidly switches on receptor oligomerization and calcium signaling. Fluorescence photographs: Low (top; scale bar, 100 μm) and high (bottom; scale bar, 10 μm) magnification views of pseudocoloured microfluorimetric images showing repeated on- and off-switching of calcium signaling in groups of cells (top) and individual cells (bottom) that precisely coincide with activation and deactivation of the magnetic field, respectively [192]. (D) Schematic of a microfluidic “alveoli-on-a-chip” that incorporates cyclic propagation of a meniscus over a flexible, PDMS membrane to recreate combined stresses found in vivo, thereby providing a more physiologic recreation of the stresses occurring in a variety of surface tension diseases. Fluorescence photographs show an impact of mechanical stress type on A549 cell morphology. Low magnification fluorescent micrographs enable visualization of cell death (red stain) and cell detachment on a membrane-wide scale. Cell detachment is visible after as few as 10 cycles of stretch, and significant cell death begins as early as 20 cycles [200].