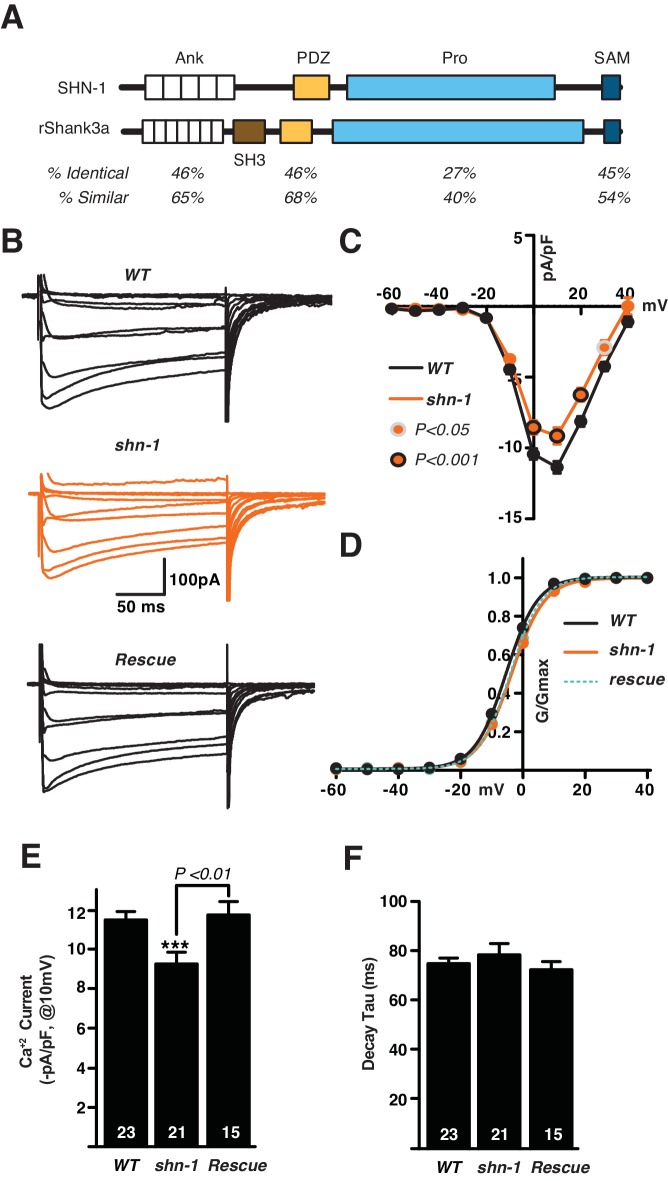
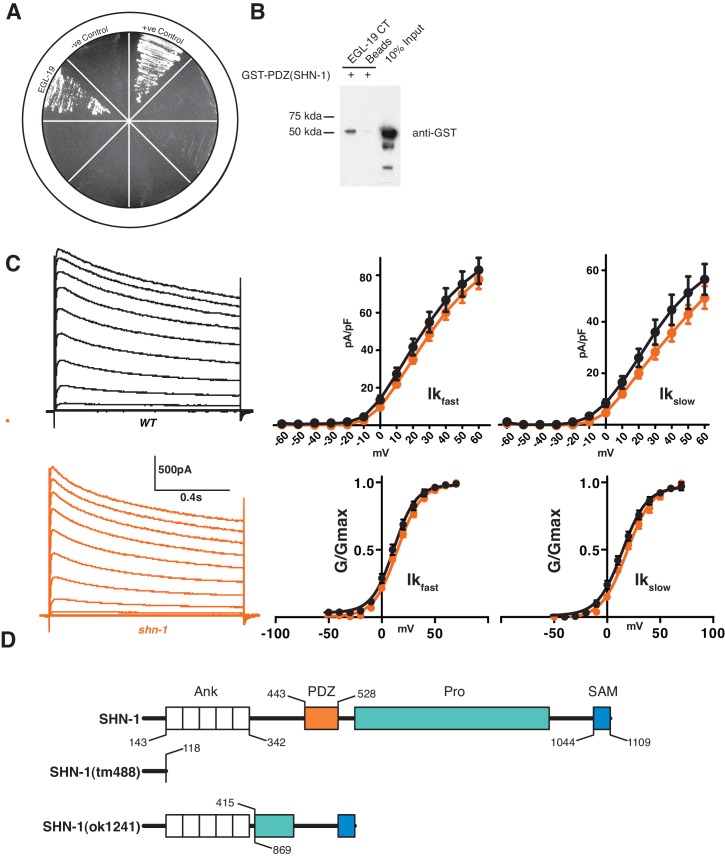
Figure 1. SHN-1 promotes EGL-19/Cav1 channel function.
(A) The protein domains found in SHN-1 and rat Shank3A are compared. SHN-1 lacks an SH3 domain but contains all other domains found in mammalian Shank proteins. Homology between the worm and mammalian protein is shown for each domain. (B–F) Voltage-activated Ca+2 currents were recorded from adult body wall muscles of the indicated genotypes at holding potentials of −60 to +40 mV. Averaged traces (B), mean current density as a function of holding potential (C), normalized conductance as a function of holding potential (D), mean current density at 10 mV (E), and mean deactivation time constants (F) are shown. shn-1 mutants had significantly decreased Ca+2 current-density and this defect was rescued by a single copy transgene expressing SHN-1 in body muscles (nuSi26) (D). No significant differences were observed for voltage-dependence of current activation and de-activation kinetics. The number of animals analyzed is indicated for each genotype. Values that differ significantly from wild type controls are indicated (***p<0.001). Error bars indicate SEM. Mean, standard errors, sample sizes, and p values for this figure are shown in Supplementary file 1.