Abstract
Pericellular proteases have long been implicated in carcinogenesis. Previous research focused on these proteins, primarily as extracellular matrix (ECM) protein degrading enzymes which allowed cancer cells to breach the basement membrane and invade surrounding tissue. However, recently, there has been a shift in the view of cell surface proteases, including serine proteases, as proteolytic modifiers of particular targets, including growth factors and protease-activated receptors, which are critical for the activation of oncogenic signaling pathways.
Of the 176 human serine proteases currently identified, a subset of 17 known as type II transmembrane serine proteases (TTSPs) [1], many have been shown to be relevant to cancer progression, since they were first identified as a family around the turn of the century. To this end, altered expression of TTSPs appeared as a trademark of several tumor types [2, 3]. However, the substrates and underlying signaling pathways remained unclear. Localization of these proteins to the cell surface places them in the unique position to mediate signal transduction between the cell and its surrounding environment. Many of the TTSPs have already been shown to play key roles in processes such as postnatal development, tissue homeostasis, and tumor progression, which share overlapping molecular mechanisms [2, 4-6].
In this review, we summarize the current knowledge regarding the role of the TTSP family in pro-oncogenic signaling.
Keywords: type II transmembrane serine proteases, cancer, matriptase, hepsin, TMPRSS2, TMPRSS4, small molecule inhibitors
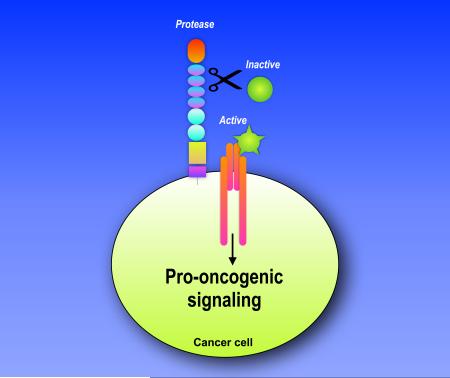
Graphical abstract
Altered expression of type II transmembrane serine proteases (TTSP) is a feature of several types of tumors. TTSPs are proteolytic modifiers of targets such as growth factors and protease-activated receptors, which are critical for the activation of oncogenic signaling pathways. Localized to the cell surface, TTSPS mediate signal transduction between the cell and its surrounding environment. Numerous studies suggest that TTSPs represent a promising option for therapeutic intervention of cancer.
Characteristics of TTSP family members
TTSPs have a single pass hydrophobic transmembrane domain near the amino terminus, which separates a short intracellular domain from a larger extracellular portion which contains a variable stem region and a C-terminal serine protease domain that has a histidine, aspartate, and serine triad of residues necessary for catalytic activity [1]. In vertebrates, this family is divided into four subfamilies: 1) matriptase, 2) hepsin/transmembrane protease/serine (TMPRSS), 3) human airway trypsin-like (HAT)/differentially expressed in squamous cell carcinoma (DESC), and 4) corin [1, 2, 7, 8]. All TTSPs are believed to be synthesized as zymogens and require activation by proteolytic cleavage. Many TTSPs, including matriptase, matriptase-2, hepsin, TMPRSS2, TMPRSS3, and TMPRSS4 are capable of auto-activation [1]. While the auto-activation mechanisms are still being elucidated, oligomerization is thought to play a role in this process [1].
Matriptase
Matriptase is among the most studied members of the TTSP family and is expressed in the epithelial compartment in a wide variety of tissues [2, 3, 9-14]. The dysregulation of matriptase is implicated in numerous cancers and associated with poor outcomes. For example, matriptase is shown to be overexpressed in a wide range of epithelial tumors (carcinomas) including of the breast, ovary, uterus, prostate, colon, cervix, and skin [1, 7, 15-17]. On the other hand some groups have described downregulation of matriptase mRNA in gastric and colorectal carcinomas [18, 19]. An important finding in several cancers is that the ratio of matriptase to its endogenous inhibitors, hepatocyte growth factor activator inhibitor (HAI)-1 and HAI-2, is increased, suggesting that the balance of protease activity can be shifted, leading to unopposed active matriptase, ultimately causing detrimental pro-carcinogenic effects. In normal tissue, the ratio is low, resulting in tightly controlled proteolytic activity [20]. However, the consequences of dysregulated matriptase become apparent in a variety of studies. For example, in a transgenic model of squamous cell carcinoma (SCC) with matriptase expression in the epidermis (K5-matriptase), all animals develop tumors. However, increasing HAI-1 or HAI-2 expression by concomitant transgenic expression abrogate tumor formation [21, 22]. In human colorectal cancer, the ratio of matriptase/HAI-1 mRNA is higher in colorectal cancer adenomas and carcinomas than corresponding tissue from control individuals [18]. During the progression of some cancers, e.g. prostate cancer the matriptase/HAI-1 ratio is observed to increase, leading to more active matriptase on the cell surface in high grade tumors [23]. In 2013, active matriptase was shown to be a functional biomarker for monitoring tumorigenesis in a mouse model of colon cancer [24].
Matriptase was first discovered in breast cancer cell lines and is highly expressed in human breast carcinoma cells [25-30]. Recently, this TTSP was shown to be critically involved in breast cancer progression through activation of the HGF/c-Met signaling axis and therefore, was identified as a potential therapeutic target in this disease (Figure 1) [25, 31].
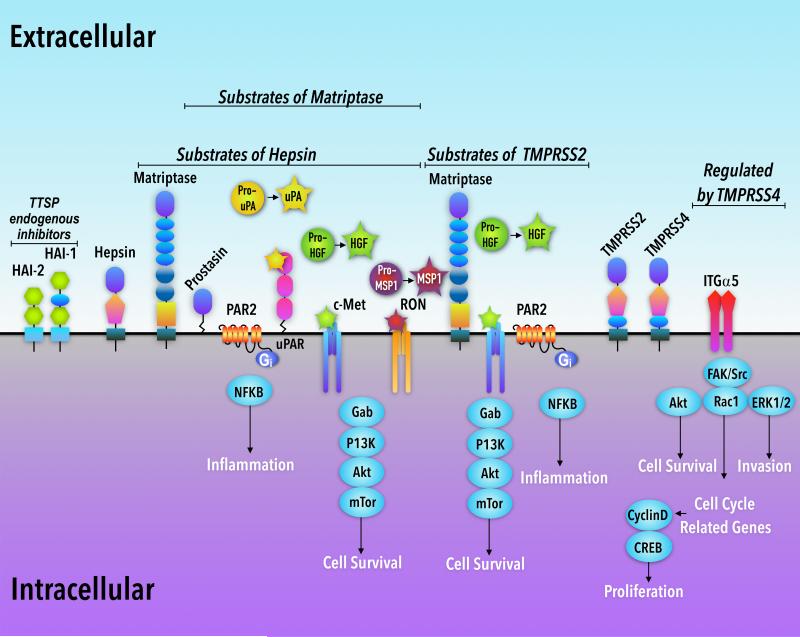
Figure 1. Proposed pro-oncogenic signaling pathways for TTSPs, matriptase, hepsin, TMPRSS2, and TMPRSS4.
The HGF/c-Met cell survival pathway is activated by matriptase, hepsin, and TMPRSS2. Similarly, matriptase and hepsin are able to cleave and activate pro-MSP-1, and pro-uPA, and activate RON, and bind to uPAR receptors, respectively. The PAR-2/NFκB pathway is activated by matriptase and TMPRSS2. TMPRSS4 is associated with signaling through the ITG-α5 pathway. Other substrates of matriptase include CDCP1/TRASK/SIMA135 (not shown) and PDGF-D (not shown) and prostasin. Matriptase was also shown to be a substrate of both hepsin and TMPRSS2. HAI-1 and HAI-2 are cell-surface endogenous inhibitors of both matriptase and hepsin
In Zoratti et al. 2015, it was demonstrated that matriptase initiates the c-Met pathway activation by cleaving and activating hepatocyte growth factor, HGF (also known as scatter factor). HGF is a pleiotropic, paracrine growth factor and key mediator of cell migration, proliferation, survival, motility and morphogenesis in epithelial cells [25, 32-34]. The inactive pro-form of HGF, which is mainly secreted by fibroblasts, binds to the c-Met tyrosine kinase receptor on the surface of breast epithelial cells. Upon proteolytic cleavage by matriptase, pro-HGF is converted to active HGF, which in turn initiates c-Met signaling and activates multiple downstream targets, including the P13/AKT pathway, and the c-Met docking protein, Gab1. Contribution of the matriptase/HGF/c-Met pathway to cancer progression was confirmed in both cell-based assays and mouse models [25]. In the study, the specific function of matriptase in mammary tumorigenesis was probed by crossing a mouse model of invasive ductal mammary carcinoma (MMTV-PymT) with mice expressing less than 25% endogenous matriptase protein (matriptase hypomorphic model). Mouse mammary tumor virus (MMTV)-Polyomavirus middle T (PymT) mice spontaneously develop multifocal mammary carcinomas with tumor progression that mirrors what is seen in human invasive ductal breast carcinomas [35, 36]. Low matriptase expression in the mammary epithelium led to a significant delay in tumor onset, tumor burden (due to decreased cancer cell proliferation), and multiplicity. Additionally, a significant abrogation of tumor progression was observed in the early stages of carcinogenesis. In 2D cell culture of human breast cancer cell lines of diverse origin, silencing of matriptase prevented activation of c-Met, Gab1, and Akt following exposure to pro-HGF suggesting that the mechanism is a general one in human breast cancer. Furthermore, in a 3D cell culture system, which more accurately mimics the microenvironment in vivo, breast cancer cells exposed to pro-HGF developed an invasive morphology (branched structures), while breast cancer cells with matriptase silenced prior to pro-HGF exposure, looked indistinguishable from controls (spheroid structures), which were not stimulated with growth factor [25].
A recent study further explored the matriptase/c-Met pathway in inflammatory breast cancer (IBC) [31]. IBC is a rare and aggressive form of invasive breast cancer, characterized by younger age of onset and lower overall survival compared to other breast cancers. Further complicating treatment is the fact that 20 – 40% of IBC cases are triple-negative breast cancers (TNBC), which excludes hormone therapy and HER2 targeting as treatment options [37]. Importantly, the matriptase/c-Met signaling axis also mediates proliferation and invasion in IBC cell lines, suggesting that targeting this pathway may be a promising new strategy in treating IBC [31].
Proteinase-activated-receptor (PAR)-2 G-protein is implicated in numerous human diseases due to the receptor's ability to induce inflammatory signaling pathways [38-42]. It is expressed by primary keratinocytes and established keratinocyte cell lines [43, 44] where activation can elicit a range of cellular responses, as well as by a variety of leukocyte populations, which infiltrate the skin during pre-neoplastic progression [38]. PAR-2 is also found in activated fibroblasts in inflammatory conditions and in endothelial cells and smooth muscle [45].
Sales et al. 2015 demonstrated that global deletion of PAR-2 from mice blocked the development of SCC of the skin in a transgenic model that expresses matriptase in the epidermis via a keratin-5 promoter (K5-matriptase transgenic mice). In PAR-2 sufficient mice, this model develops spontaneous multistage SCC, with invasive lesions beginning around one year of age [21]. However, mice lacking PAR-2 were histologically indistinguishable from wild-type littermates demonstrating that matriptase-mediated pre-malignant progression is PAR-2 dependent [38].
In a separate model of Ras-dependent SCC in K5-matriptase transgenic mice, induced by exposure to the chemical carcinogen, 7,12-Dimethylbenz[a]anthracene (DMBA), ablation of PAR-2 impeded matriptase-mediated potentiation of SCC, leading to decreased tumor latency [38].
The same group previously showed that in the matriptase-driven SCC model, recruitment of inflammatory cells including mast cells to the underlying dermis [21] precedes SCC development and the newer study builds upon this observation. In cell-based assays, PAR-2 activation by matriptase, caused induction of nuclear factor (NF)κB through the Gαi receptor and the associated release of NFκB-dependent inflammatory cytokines. Importantly, specific deletion of PAR-2 from bone-marrow derived hematopoietic stem cells did not affect matriptase-mediated pre-malignant progression when transplanted to K5-matriptase transgenic mice, suggesting that dysregulated matriptase activates keratinocyte PAR-2 to promote pro-inflammatory and pro-tumorigenic effects [38]. As PAR-2 and matriptase have been shown to be consistently co-expressed in the epithelial compartment of human SCC [46], this is strong evidence that a matriptase–PAR-2 signaling axis contributes to human disease.
As seen in breast cancer, pro-HGF activation is a critical component through which matriptase exerts promotion of SCC, and matriptase fails to drive tumor progression in mice with specific deletion of c-Met from the epidermal basal cell keratinocyte compartment [47]. In SCC, activation of both the HGF/c-Met and PAR-2 coupled signaling pathway is involved in matriptase-mediated pro-tumorigenic effects. It was shown that the serine-threonine kinase, mTor, is an essential component of matriptase-mediated HGF/c-Met signaling. Furthermore, mTor activation was required for matriptase/c-Met-induced SCC, as K5-matriptase mice treated with the mTor inhibitor, rapamycin, remained tumor-free. [47].
Additionally, it was shown that as with PAR-2, c-Met deficiency in the skin of K5-matriptase mice abrogates the ability of matriptase to potentiate DMBA-induced tumorigenesis. Unlike, PAR-2 deletion, however, loss of c-Met does not prevent inflammatory cell accumulation as a result of dysregulated matriptase in mice. Therefore, it would appear that matriptase induces activation of two separate pro-tumorigenic pathways (c-Met-Akt-mTor and PAR-2-NFκB), both of which are required for SCC promotion. Sales et al. 2015 highlight that an intriguing, unanswered question that arises from these studies is why matriptase-induced inflammatory PAR-2 signaling does not promote malignant progression in the absence of activated c-Met and why, conversely, matriptase-induced c-Met-Akt signaling does not promote malignant progression in the absence of activated PAR-2. These in vivo findings were accompanied by cell culture studies where no evidence was found that PAR-2 activation was associated with increased c-Met activity in cell-based assay using HEK293 cells or immortalized keratinocytes [38].
The transcriptional profiling of nearly 2000 human samples, which included normal tissues, cancer cell lines, and cancer tissue, as well as biochemical enzyme characterization found matriptase to be part of a signaling pathway that includes the growth factor, macrophage stimulating protein 1 (MSP-1), and its corresponding receptor, RON (Recepteur d'Origine Nantais) [48]. Cell-based experiments confirmed this interaction: on peritoneal macrophages, cell surface bound matriptase activates MSP-1 into its active form, which then binds to and activates RON-mediated signaling. Activation of RON led to changes in cell morphology as well as downstream biochemical changes, such as modulation of NO production in bone-derived macrophages [48]. While the matriptase/RON interaction has been previously described in macrophages as affecting activation, chemotaxis, and proliferation [49], RON has also received attention for its role in cancer pathogenesis. For example, transgenic mice expressing RON in distal lung epithelial cells under the surfactant protein C promoter, develop multiple lung tumors in which RON was highly expressed and constitutively active [50].
Matriptase has been shown to cleave and activate the pro-form of the urokinase plasminogen activator (uPA) [51-54] and it has been suggested that matriptase promotes progression of cancer through this activation. Thus, it was proposed that inhibition of ovarian cancer cell invasion by RNAi-mediated down-regulation of matriptase occurs through suppression of activation of uPA receptor (uPAR) -bound pro-uPA [55]. In a recent study, matriptase was not found to be a critical activator of pro-uPA in vivo in the MMTV-PymT mouse model of breast cancer [25]. Western blot analysis of whole tissue lysates of mammary tumors from matriptase hypomorphic mice and control, detected uPA in both its pro-form and two-chain active form, with no difference in the pro-uPA/active uPA ratio [25].
The SRC-associated protein CUB-domain-containing protein 1 (CDCP1), also referred to as TRASK and SIMA135, is an integral membrane glycoprotein that has been shown to be upregulated in several kinds of malignancies, including of the breast, colon, and lung [56]. Matriptase and CDCP1 mRNA have correlated expression in many tissue types [48] and matriptase has been shown to both interact with and cleave CDCP1 in in vitro experiments [57]. This was corroborated by another study that showed that matriptase was sufficient, but not essential to process CDCP1 in Hela cells and 22Rv1 prostate cancer cells [58]. However, silencing of matriptase in prostate cancer cells lines did not reduce CDCP1 processing [58].
Members of the platelet-derived-growth-factor (PGDF) family are known to induce cell-migration, proliferation, and malignant transformation upon binding to their receptors (PDGF receptor α and β) [59]. In particular, β-PDGFR-mediated signaling is thought to be involved in prostate cancer and PDGF-D as a ligand for β-PDGFR was discovered in prostate cancer cells [60]. The same study showed that in these cells, matriptase activates PDGF-D and can deactivate the resulting growth-factor through further proteolytic cleavage [60].
While this review focuses on the signaling pathways underlying TTSP function and their contribution to cancer pathogenesis, the way in which TTSPs influence the tumor microenvironment should also be considered. The ECM is crucial for the maintenance of tissue homeostasis [61] and a loss of ECM integrity contributes to cancer cell growth and invasion [62, 63]. Cancer cells are known to secrete proteolytic enzymes to degrade ECM and invade surrounding tissue [64]. Recently, TMPRSS2 was shown to activate matriptase (described in the TMPRSS2 section), and in a xenograft model of prostate cancer, the TMPRSS2 protein level was shown to correlate with activated matriptase and degradation of the ECM components, nidogen-1 and laminin β1 [65]. It should be mentioned that direct cleavage of nidogen and laminin β1 by TMPRSS2 was demonstrated whereas no data on the ability of matriptase to do the same was presented [65]. In another study, active matriptase was shown to degrade ECM components fibronectin and laminin, in vitro [66].
While there is some evidence for TTSPs influencing ECM integrity either directly through cleaving ECM components, or indirectly through secondary pathways, further exploration in in vivo models of cancer is required.
Hepsin
Hepsin is shown to be consistently expressed and upregulated in prostate cancer (reviewed in [67, 68]) and high levels in the tumor are indicative of poor outcome and relapse after radical prostatectomy [67, 68]. A search for genetic factors, which may contribute to prostate cancer susceptibility in men of European origin, identified several single nucleotide polymorphisms (SNPs) in non-coding regions of the hepsin gene, however the effect of these SNPs on hepsin expression and activity remains unknown [2, 69, 70]. Similar correlations were found in a population of Korean men [71].
Upregulation of hepsin in the prostate epithelium of mice causes disorganization of the basement membrane (BM) of the prostate in probasin (PB) promoter-driven hepsin transgenic mice. When these animals are crossed into transgenic mice carrying the SV40 large T antigen (Tag) under the control of the large probasin (LPB) promoter, a non-metastatic model of prostate cancer [72], metastasis to distant organs including liver, lung, and bone occurs [73]. The authors suggest that hepsin likely exerts its effects through proteolytic modification of the ECM or through the activation of other proteases.
Hepsin expression has been well-documented in several other types of epithelial cancers, including ovarian, and breast [2, 74-76]. When SKOV3 and C11 human ovarian cancer cells with forced expression of hepsin (through lentiviral transduction) are injected into the flank of female nude mice, tumor growth is promoted. The ability of ectopic hepsin to induce tumor growth is dependent on the catalytic activity of the protein, as mutation of the catalytic triad residues abates tumor progression [75].
Although the presence of hepsin in many tumor types and its ability to promote tumor growth in animal models suggests that its activity may confer oncogenicity, the underlying cellular signaling is still actively being investigated. Several substrates have been identified in cell-free or cell-based assays; these include the pro-forms of HGF and MSP-1 [77-79]. Cleavage by hepsin converts these proteins into their active forms which are then able to activate their cognate receptors, c-Met and RON, respectively [77-79]. Additionally, hepsin cleaves zymogens of other cell-surface associated serine proteases in vitro, such as matriptase [40], pro-uPA [80], and prostasin, a glycophosphatidylinositol-anchored serine protease [81]. Prostasin is also known to be a substrate of matriptase [11, 82, 83].
Elevated hepsin levels promote epithelial carcinogenesis potentially though basement membrane (BM) and ECM degradation and remodeling, but the molecular underpinnings are still under study. Deregulated hepsin expression is found to be sufficient to disrupt epithelial integrity in primary mouse mammary epithelial cultures [84]. Hepsin is also reported to be the culprit underlying the defective cell polarity, tight junction positioning, desmosomal integrity as well as BM fragmentation observed in primary epithelial cells and tumors from liver kinase B1 (LKB1) deficient mice crossed with oncogenic c-Myc mice [84].
Tervonen et al. showed that hepsin was overexpressed in more than 40% of breast cancers in tumor microarrays. Hepsin was strongly expressed in 40–50% of luminal A, B, and HER2+ subtypes and up to 60% of TNBCs. Furthermore, hepsin was predominantly expressed as the processed active form, as observed by western blot in five out of seven primary breast cancer samples [74].
Potentiation by hepsin of mammary tumor development was also shown in the transgenic Wap-c-Myc mouse model. In these mice, pregnancy induces activation of the Wap promoter to increase levels of c-Myc expression in the mammary gland which induces mammary carcinomas. Upregulation of endogenous hepsin is not observed in this model and was used to elucidate the effect of transgenic hepsin expression on mammary tumor development. Transplanted primary mammary epithelial cells were isolated from donor Wap-Myc mice, followed by transplantation of the cells to cleared fat pads of syngeneic wild-type virgin hosts. Lentivirus mediated inducible hepsin expression of the primary cells led to decreased latency of tumor formation. Thus, expression of hepsin decreased latency to 189 days compared to over 260 days for mice expressing catalytically inactive hepsin [74]. These data suggest that hepsin may influence tumor initiation and early progression phases.
HGF/c-Met signaling represents a potential pathway by which hepsin contributes to tumorigenesis. In MCF10A mammary epithelial cells, overexpression of hepsin enables cells to proteolytically activate pro-HGF, leading to activation of the c-Met pathway. Zoratti et al. also described the HGF/c-Met axis as the target of matriptase in a variety of breast cancer cell lines. However, in these lines, matriptase appeared to be the primary activator, since no or minimal residual pro-HGF activity was detected following matriptase silencing. Therefore, perhaps there are different predominant TTSP initiators of c-Met/HGF signaling depending on cell type and context. MCF10A cells differ from the cells examined by Zoratti et al. in that they are not breast cancer cells. Instead they are typically used as a model for normal breast cells. MCF10As were derived from benign proliferative tissue and spontaneously immortalized without defined factors [85, 86]. Additionally, Tervonen et al. explored the effects of overexpressed hepsin which appears to allow for these cells to proteolytically activate pro-HGF, while Zoratti et al. explored the role of silencing endogenous matriptase levels [25]. It was found that the consequences of hepsin overexpression in MCF10A cells include down-modulated demosomal, hemidesmosomal and basal lamina proteins, which damage epithelial cohesion and sites which connect the basal surface of these cells to the basement membrane. It should also be noted that induced hepsin acutely down-modulated its cognate inhibitor, HAI-1 [74]. HAI-1 and HAI-2 have been shown to inhibit hepsin activity in cell-free enzyme inhibition assays [79]. These experiments are consistent with a model of hepsin oncogenic activity, in which increased expression of hepsin and loss of HAI-1 contribute to augmented HGF/c-Met signaling.
TMPRSS2
Like hepsin in prostate cancer, TMPRSS2 and TMPRSS4 may also promote metastasis. TMPRSS2 message is most highly expressed in the epithelium of prostate tissue, with pancreas, kidney, and colon exhibiting strong expression, and lung, small intestine, and liver exhibiting weak expression [87]. TMPRSS2 gene expression is several fold higher in cancer cells compared to benign cells in human specimens containing cancerous and benign prostate tissue [88]. Additionally, chromosomal rearrangements of the TMPRSS2 gene have been identified in prostate cancer patients, in which 5′ untranslated region of the gene, containing androgen-responsive elements, becomes fused to coding sequences of transcription factors from the E26 transformation-specific (ETS) family, ERG, ETV1, or ETV4, thereby making their expression androgen-inducible [89-91]. In fact, ERG and ETV1 are fused with TMPRSS2 in approximately 50–79% cases of prostate cancer [92] and TMPRSS2–ETS fusion is associated with a poor prognosis in localized prostate cancer [93].
TMPRSS2 message is upregulated in androgen-dependent prostate cancer compared to normal prostate epithelium or benign hyperplasia [2, 88, 94], and TMPRSS2 transcription is regulated by androgenic ligands and the androgen receptor (AR) [94]. In the transgenic adenocarcinoma of the mouse prostate (TRAMP) model, mice spontaneously develop autochthonous prostate tumors following the onset of puberty [95]. Deletion of TMPRSS2 in TRAMP mice significantly attenuated metastasis despite increased primary tumor size [96]. This is in line with the emerging view that metastasis and primary tumor growth are controlled by different factors [97] and suggests that TMPRSS2 may be critical in tumor metastatic behavior, specifically. Since the vast majority of prostate cancer associated deaths are due to metastasis, this is of particular importance.
While the relationship between increased gene message or protein expression of TMPRSS2 in prostate cancer aggression is well-established, the signaling underlying its oncogenic role is less clear. In 2005, it was shown that TMPRSS2 is able to cleave and activate PAR-2 on human prostate cancer cells (LNCaP) [98]. A recent study also identified matriptase as a possible substrate of TMPRSS2. Thus, stably transfected TMPRSS2-overexpressing clones from a variety of prostate cancer cell lines showed increased levels of activated matriptase, which formed a 120-kDa complex with HAI-1 and corresponded with reduced levels of latent matriptase (70 kDa) and free HAI-1. These data were further corroborated by the finding that orthotopic grafts of LNCaP cells overexpressing TMPRSS2 increased both active matriptase and metastases, and that this increase is dependent upon TMPRSS2 catalytic activity [15, 65]. High-throughput screens of combinatorial libraries also identified pro-HGF as a substrate and it was confirmed that exogenous pro-HGF activated by incubation with TMPRSS2 was sufficient to activate the c-Met receptor, expressed in DU145 prostate cancer cells. Additionally, LNCaP and C4-2B prostate cancer cell lines which endogenously express TMPRSS2, were shown to be more invasive upon exposure to pro-HGF compared to vehicle or pro-HGF plus an HGF-neutralizing antibody [96].
TMPRSS4
TMPRSS4 was first identified in pancreatic cancer [99]. It is now known to also be overexpressed in ovarian, thyroid, colorectal, breast, cervical, gallbladder, gastric, and liver cancer [100, 101]. However, the mechanism through which levels are increased, for example by gene amplification, chromosomal rearrangement or transcriptional dysregulation is still unclear [100].
Silencing of this TTSP in thyroid cancer cells suppresses proliferation which correlates with decreased cyclin D1 mRNA levels and inhibition of Ser133 phosphorylation of the cyclin D1 transcription factor, CREB [102]. In lung and colon cancer cells TMPRSS14 knock-down impedes migration [103, 104] and invasion through a variety of extracellular matrices and impairs cell proliferation [103], while overexpression enhances migration and invasion in colon cancer [103, 104]. A more recent study reported that TMPRSS4 induces Slug and cyclin D1 through activator protein-1 (AP-1) activation in PC3 cancer cells, leading to invasion and proliferation [105]. Additionally, the authors describe a positive feedback loop between Slug and AP-1, which leads to the induction of cyclin D1 and cell proliferation [105].
Like TMPRSS2, TMPRSS4 appears to enhance metastatic potential, possibly through promoting acquisition of epithelial to mesenchymal transition (EMT) (see [103, 104]). TMPRSS4-overexpression in SW480 (colon cancer) cells causes increased invasion, in vitro, and increased metastasis to the liver when injected intrasplenically into nude mice, [103]. While this phenotype is attributed to loss of e-cadherin and EMT, the underlying signaling mechanism has yet to be elucidated. Of the several well-known e-cadherin transcriptional repressors/EMT-inducing transcriptional repressors examined, there was no detectable upregulation of SNAIL, SLUG or TWIST mRNA, although there was concomitant induction of SIP1/ZEB2, which are known to repress e-cadherin, in response to TMPRSS4 overexpression [103]. Studies in lung cancer cell- and animal-based models revealed that TMPRSS4 silencing resulted in a significant reduction in proliferation, clonogenic capacity, and invasion. In addition, a significant impairment of lung colonization and growth was found when mice were tail vein injected with TMPRSS4 silenced lung cancer cells [104].
Further studies in SW480 cells showed that overexpression of TMPRSS4 induces a signaling cascade which includes FAK, ERK1/2, Akt, Src, and Rac1 activation and identified upregulation of integrin α5 as a potential mechanism through which TMPRSS4 induces signaling transduction, invasion, and EMT [106]. However, it is not yet known how the protease regulates integrin expression. In support of a link between TMPRSS4 and integrin α5, transcriptomic profiling studies of TMPRSS4-silenced lung cancer cells, revealed that the gene, MIR205HG coding for the metastasis suppressing micro-RNA, mir-205 increases upon TMPRSS4 knock-down in lung cancer cells and integrin α5 was shown to be a direct target of this micro-RNA [107]. Therefore, a new regulatory pathway was proposed involving TMPRSS4/miR-205/integrin α5.
Although the study of TMPRSS4 has gained momentum in recent years, genetic animal models are necessary to fully unravel the role of the signaling pathways highlighted here. TMPRSS4 null mice were generated in 2015 [108] which are viable, fertile, and did not show any obvious abnormalities. However, at the time this review was written, no groups had reported the use of this model in cancer studies.
TTSPs as novel therapeutic targets in cancer
Although further validation is required in animal models for some of the TTSPs discussed in this review, in general TTSPs represent a viable target for the development of therapeutic agents. Protein-based targeting agents, including antibodies and modified cognate inhibitors, as well as small molecule inhibitors have been shown to effectively block activity of several TTSPs in in vitro or in vivo settings, and inhibit some aspects of cancer pathogenesis in cell and animal models.
Thus far, early studies targeting matriptase appear to be promising. As proof of principle, matriptase-mediated tumorigenesis was shown to be negated by its cognate inhibitors, HAI-1 and HAI-2. Transgenic expression of either HAI-1 or HAI-2, not only prevents malignant transformation in the SCC model of transgenic mice overexpressing matriptase in the epidermis [21, 22] but importantly, also causes already established tumors to regress demonstrating the power of utilizing this pathway as a means of intervention [22].
A number of small molecule inhibitors targeting matriptase have been described [52, 109-115]. IN-1, which contains a ketobenzothiazole serine trap was designed based on the auto-activation site (RQAR) of matriptase [116]. IN-1 is highly selective for matriptase compared to other related proteases [116]. This compound was shown to efficiently block pro-HGF conversion to active HGF, subsequent activation of c-Met, Gab1, and AKT, and therefore, cell proliferation and invasion in both murine primary mammary carcinoma cells and in human breast cancer cell lines [25]. This same compound was shown to block the aforementioned signaling pathway in human IBC cell lines and inhibit cell proliferation [31].
In 2014, the first substrate-based ketothiazole inhibitors of substrate-based ketothiazole inhibitors of hepatocyte growth factor activator (HGFA), matriptase and hepsin were described. The compounds blocked the conversion of native pro-HGF and pro-MSP by HGFA with equal potency and caused a dose-dependent decrease of c-Met signaling in MDA-MB-231 breast cancer cells [117]. Recently, the optimization of α-ketothiazole inhibitors into selective and potent inhibitors that display sub-nanomolar enzyme inhibition against one, two, or all three of HGFA, matriptase, and hepsin, were described [118]. These inhibitors target activation of pro-HGF and pro-MSP. Further, they blocked pro-HGF-mediated migration of invasive DU145 prostate cancer cells [118]. Around the same time, a study by a different group described the development of a triplex benzamide micromolar inhibitor of matriptase, hepsin, and HGFA, called SRI 2315 [119]. This compound was shown to inhibit fibroblast-induced c-Met activation, EMT, and migration of cancer cells. It also overcame resistance to cetuximab and gefitinib in HGF-producing colon cancer cells as well as averted fibroblast-mediated resistance to epidermal growth factor receptor (EGFR) inhibitors [119].
MCoTI-II, a cyclic microprotein of the squash Momordica cochinchinensis trypsin-inhibitor family, is a specific and potent matriptase inhibitor which blocks pro-HGF activation in cell free and cell culture models and was shown to selectively inhibit invasion of matriptase-expressing prostate cancer cells [115]. Additionally, CVS-3983, another selective small molecule matriptase inhibitor was found to suppress the growth of androgen independent human prostate tumor xenografts. [112]
Hypomorphic matriptase mice, which retain <1% of both intestinal [120] and epidermal matriptase message [121] have only mild icthyosis of the skin, which subsides with age [121], and a slight intestinal barrier defect that has no apparent adverse consequences in adult mice [120]. Also, humans with a hypomorphic mutation in the matriptase-encoding St14 gene resulting in a matriptase mutant protein with 1000-fold lower activity than that of wild-type matriptase, have only a mild and temporary ichthyosis of the skin, brittle hair, but no reported gastrointestinal symptoms [121, 122]. Consequently, drugs which target the matriptase pathway are not anticipated to cause significant side-effects.
Drugs developed against c-Met receptor include several which are currently in phase 2 clinical trials in patients with TNBC, including IBC. Some of these specifically target c-Met only, while others target a combination of c-Met and other kinases: XL184 (cabozantinib), c-Met-vascular endothelial growth factor receptor 2 (VEGFR2) inhibitor, and X-396 and X-376 (crizotinib), c-Met- anaplastic lymphoma kinase (ALK) inhibitor. Combining targeted therapy to multiple pathways, including TTSPs may prevent resistance to kinase inhibitors [123].
The development and use of selective hepsin inhibitors is still in progress, however a few have been tested in cell-based and animal models. An analysis of small molecule hepsin inhibitors based on benzamidine and benzguanidine arginine mimetics revealed a subset of piperazinyl ureas that had potency and selectivity for hepsin over matriptase and HGFA in enzymatic assays [124]. In 2014, Tang et al. reported the development of a novel, non-toxic, and orally bioavailable small molecule hepsin inhibitor, HepIn-13 [125]. This inhibitor can block pro-HGF conversion to active HGF by hepsin, in an overexpression system in HEK293 cells and displayed minimal inhibition of matriptase (the effect on other related serine proteases was not reported). In the double transgenic line, LPB-Tag/PB-Hepsin mice, which develops metastasis [73], HepIn-13 reduced metastasis to the lungs (42% of control group versus 20% of treatment group).
In a xenograft model of prostate cancer, in which LnCap-34 cells display a hepsin-mediated ability to invade and develop lymph node metastasis, the HAI-1 protein-based hepsin inhibitor; a PEGylated form of the Kunitz domain-1 (KD1-PEG), decreased contralateral prostate invasion and lymph node metastasis by 50% upon daily intraperitoneal administration [126]. The authors cautioned that inhibitory effects of KD1-PEG on other proteases cannot formally be ruled out.
In addition to these inhibitors, studies using antibodies targeting hepsin have also been reported. The monoclonal, humanized, allosteric antibody, hH35 inhibits hepsin enzymatic activity at nanomolar concentrations [127]. Fab25, which was found by screening a Fab phage display library, is another allosteric inhibitor [78], although their effects in either cell- or animal-based systems remain unexplored. Xuan et al. generated monoclonal neutralizing antibodies that inhibited hepsin's proteolytic activity and significantly reduced invasion of DU145 (protaste cancer) and CAOV-3 (ovarian cancer) cells in in vitro assays [128].
Although not a specific inhibitor, WX-UK1, which was originally developed against uPA is a micromolar range inhibitor of the hepsin active site. Currently under clinical investigation as an oral pro-drug, WX-671/MESUPRON, for the treatment of pancreatic cancer and metastatic breast cancer [129], this drug was also found to be effective in suppressing hepsin activity in MCF10A hepsin overexpressing cells [74]. Additionally, WX-UK1 demonstrated antitumor activity in a 4T1 orthotopic breast tumor model. 4T1 cells express both zymogen and processed forms of hepsin, and WX-UK1 inhibited the growth of 4T1 tumors in syngeneic mice as efficiently as the standard-of-care drug tamoxifen [74].
It has been shown that hepsin is not an essential gene and knockout mice are viable and fertile [130, 131], although they do exhibit hearing loss due to developmental deformities in the cochlea [132], enlarged hepatocytes, and narrowed liver sinusoids [133]. Further, mice treated with the HepIn-13 inhibitor did not develop any apparent deficiencies [125], suggesting that targeting this TTSP would not cause any major detrimental side effects in humans, although more rigorous studies are needed.
Regarding TMPRSS2, a chemical library screen identified bromhexine hydrochloride, an FDA-approved ingredient in mucolytic cough suppressants, as a bioavailable drug suppressing distant metastasis to lung and liver sites in TRAMP mice [96]. Other synthetic inhibitors have been identified, although their mechanism is unclear [15, 134, 135]. Deletion of TMPRSS2 from mice does not appear to affect development or physiological function, suggesting that ablation of TMPRSS2 in cancer may have minimal side effects [136].
For TMPRSS4, a novel series of 2-hydroxydiarylamide derivatives were synthesized and evaluated for inhibiting TMPRSS4 protease activity and suppressing cancer cell invasion. These derivatives exhibited inhibitory activity against the TTSP and correlated with anti-invasive activity against colon cancer cells overexpressing TMPRSS4. However, the selectivity of this inhibitor was not reported [137].
Conclusions
Recent work has established a clear role for TTSPs in cancer pathogenesis in a variety of carcinomas. As the pro-oncogenic signaling is elucidated, recurrent signaling themes have emerged. The HGF/c-Met and PAR-2 signaling pathways link most of the TTSPs discussed in this review and it was recently shown that concurrent activation of both of these pathways by matriptase is required for development of SCC, underscoring the relationship between inflammatory and cell-survival signaling in cancer development [38]. The HGF/c-Met pathway can be activated by matriptase, hepsin and TMPRSS2 and it is likely that there is a degree of functional overlap between the proteases in vivo that is influenced by multiple factors including tissue/cancer type as well as genetic and epigenetic determinants. While matriptase and hepsin signaling through the RON receptor has also been described in cell-based experiments, it has yet to be validated in animal models. Studies have also shown that the TTSPs may interact with each other: matriptase, which can auto-activate, may be a substrate of both hepsin and TMPRSS2, and HAI-1/2 are endogenous inhibitors of matriptase and cognate inhibitors of hepsin, suggesting complex interaction between TTSPs and respective downstream signaling cascades. Many TTSPs, especially within the HAT/DESC/HATL and matriptase subfamilies, display a high degree of similarity in both their primary sequence and domain structure. However, it remains to be seen if this translates into overlapping biological functions in tissues with overlapping TTSP expression or with respect to cancer and other diseases.
In sum, TTSPs represent a promising option for therapeutic intervention and numerous studies on the development of inhibitors against TTSP-mediated signaling have been reported, although no specific TTSPs inhibitors are currently in clinical trials. Importantly, based on previous experiments, targeting of matriptase, hepsin, or TMPRSS2 is not anticipated to cause major adverse effects. It is the hope that the TTSP basic science and drug discovery work performed in laboratories across the world, a small fraction of which is described above, will provide a springboard for future clinical applications using TTSPs as targets in cancer intervention.
Acknowledgements
This work was supported by The American Cancer Society PF- 14-168-01-CSM (L.M.T) and NIH/NCI NCI RCA60565A grant (K.L.). We were not able to include all original published work performed by dedicated researchers in the field due to space restraints.
Abbreviations
- TTSP
type II transmembrane serine protease
- TMPRSS
transmembrane protease, serine
- HAT
human airway trypsin-like
- DESC
differentially expressed in squamous cell carcinoma
- HGF
hepatocyte growth factor
- SF
scatter factor
- HAI-1
hepatocyte growth factor inhibitor-1
- HAI-2
hepatocyte growth factor inhibitor-2
- SCC
squamous cell carcinoma
- MMTV
mouse mammary tumor virus
- PymT
polyomavirus middle T
- CDCP1
CUB-domain-containing protein 1
- PGDF-D
platelet derived growth-factor-D
- IBC
inflammatory breast cancer
- PAR-2
proteinase-activated-receptor-2
- DMBA
dimethylbenzanthracene
- K5
keratin-5
- NF
nuclear factor
- PB
probasin
- Tag
T antigen
- LPB
large probasin
- ECM
extracellular matrix
- BM
basement membrane
- LKB1
liver kinase B1
- E26
transformation-specific
- ETS pro-UPA
pro-urokinase type plasminogen activator
- AP-1
activator protein-1
- AR
androgen receptor
- TRAMP
transgenic adenocarcinoma of the mouse prostate
- HGFA
hepatocyte growth factor activator
- EMT
epithelial to mesenchymal transition
- MSP-1
macrophage stimulating protein 1
- RON
Recepteur d'Origine Nantais
- TNBC
triple-negative breast cancer
- ITG
integrin
- SNPs
single nucleotide polymorphisms
- KD1-PEG
PEGylated form of the Kunitz domain-1
- EGFR
epidermal growth factor receptor
- VEGFR2
vascular endothelial growth factor receptor 2
- ALK
anaplastic lymphoma kinase
References
- 1.Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–81. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szabo R, Bugge TH. Type II transmembrane serine proteases in development and disease. Int J Biochem Cell Biol. 2008;40:1297–316. doi: 10.1016/j.biocel.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Uhland K. Matriptase and its putative role in cancer. Cell Mol Life Sci. 2006;63:2968–78. doi: 10.1007/s00018-006-6298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb SL, Sanders AJ, Mason MD, Jiang WG. Type II transmembrane serine protease (TTSP) deregulation in cancer. Front Biosci (Landmark Ed) 2011;16:539–52. doi: 10.2741/3704. [DOI] [PubMed] [Google Scholar]
- 5.Antalis TM, Bugge TH, Wu Q. Membrane-anchored serine proteases in health and disease. Prog Mol Biol Transl Sci. 2011;99:1–50. doi: 10.1016/B978-0-12-385504-6.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo R, Bugge TH. Membrane-anchored serine proteases in vertebrate cell and developmental biology. Annu Rev Cell Dev Biol. 2011;27:213–35. doi: 10.1146/annurev-cellbio-092910-154247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J. 2010;428:325–46. doi: 10.1042/BJ20100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem. 2001;276:857–60. doi: 10.1074/jbc.R000020200. [DOI] [PubMed] [Google Scholar]
- 9.Fan B, Brennan J, Grant D, Peale F, Rangell L, Kirchhofer D. Hepatocyte growth factor activator inhibitor-1 (HAI-1) is essential for the integrity of basement membranes in the developing placental labyrinth. Dev Biol. 2007;303:222–30. doi: 10.1016/j.ydbio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 10.List K, Szabo R, Molinolo A, Nielsen BS, Bugge TH. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. Am J Pathol. 2006;168:1513–25. doi: 10.2353/ajpath.2006.051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, Antalis TM, Bugge TH, List K. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 2006;281:32941–5. doi: 10.1074/jbc.C600208200. [DOI] [PubMed] [Google Scholar]
- 12.Oberst MD, Singh B, Ozdemirli M, Dickson RB, Johnson MD, Lin CY. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem. 2003;51:1017–25. doi: 10.1177/002215540305100805. [DOI] [PubMed] [Google Scholar]
- 13.Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26:1546–56. doi: 10.1038/sj.onc.1209966. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi T, Shuman MA, Craik CS. Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc Natl Acad Sci U S A. 1999;96:11054–61. doi: 10.1073/pnas.96.20.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray AS, Varela FA, List K. Type II transmembrane serine proteases as potential targets for cancer therapy. Biol Chem. 2016;397:815–26. doi: 10.1515/hsz-2016-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.List K, Bugge TH, Szabo R. Matriptase: potent proteolysis on the cell surface. Mol Med. 2006;12:1–7. doi: 10.2119/2006-00022.List. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.List K, Kosa P, Szabo R, Bey AL, Wang CB, Molinolo A, Bugge TH. Epithelial integrity is maintained by a matriptase-dependent proteolytic pathway. Am J Pathol. 2009;175:1453–63. doi: 10.2353/ajpath.2009.090240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel LK, Saebo M, Skjelbred CF, Abell K, Pedersen ED, Vogel U, Kure EH. The ratio of Matriptase/HAI-1 mRNA is higher in colorectal cancer adenomas and carcinomas than corresponding tissue from control individuals. BMC Cancer. 2006;6:176. doi: 10.1186/1471-2407-6-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng L, Cao J, Zhang X. Expression of serine protease SNC19/matriptase and its inhibitor hepatocyte growth factor activator inhibitor type 1 in normal and malignant tissues of gastrointestinal tract. World J Gastroenterol. 2005;11:6202–7. doi: 10.3748/wjg.v11.i39.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parr C, Sanders AJ, Jiang WG. Hepatocyte growth factor activation inhibitors - therapeutic potential in cancer. Anticancer Agents Med Chem. 2010;10:47–57. doi: 10.2174/1871520611009010047. [DOI] [PubMed] [Google Scholar]
- 21.List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, Burke B, Nielsen BS, Gutkind JS, Bugge TH. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–50. doi: 10.1101/gad.1300705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sales KU, Friis S, Abusleme L, Moutsopoulos NM, Bugge TH. Matriptase promotes inflammatory cell accumulation and progression of established epidermal tumors. Oncogene. 2015;34:4664–72. doi: 10.1038/onc.2014.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB, Reagan-Shaw S, Jarrard DF, Mukhtar H. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev. 2006;15:217–27. doi: 10.1158/1055-9965.EPI-05-0737. [DOI] [PubMed] [Google Scholar]
- 24.LeBeau AM, Lee M, Murphy ST, Hann BC, Warren RS, Delos Santos R, Kurhanewicz J, Hanash SM, VanBrocklin HF, Craik CS. Imaging a functional tumorigenic biomarker in the transformed epithelium. Proc Natl Acad Sci U S A. 2013;110:93–8. doi: 10.1073/pnas.1218694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoratti GL, Tanabe LM, Varela FA, Murray AS, Bergum C, Colombo E, Lang JE, Molinolo AA, Leduc R, Marsault E, Boerner J, List K. Targeting matriptase in breast cancer abrogates tumour progression via impairment of stromal-epithelial growth factor signalling. Nat Commun. 2015;6:6776. doi: 10.1038/ncomms7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatt AS, Takeuchi T, Ylstra B, Ginzinger D, Albertson D, Shuman MA, Craik CS. Quantitation of membrane type serine protease 1 (MT-SP1) in transformed and normal cells. Biol Chem. 2003;384:257–66. doi: 10.1515/BC.2003.029. [DOI] [PubMed] [Google Scholar]
- 27.Lin CY, Wang JK, Torri J, Dou L, Sang QA, Dickson RB. Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells. Monoclonal antibody production, isolation, and localization. J Biol Chem. 1997;272:9147–52. [PubMed] [Google Scholar]
- 28.Oberst M, Anders J, Xie B, Singh B, Ossandon M, Johnson M, Dickson RB, Lin CY. Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol. 2001;158:1301–11. doi: 10.1016/S0002-9440(10)64081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin JS, Cheng TF, Tsai WC, Sheu LF, Chiang H, Yu CP. Expression of the serine protease, matriptase, in breast ductal carcinoma of Chinese women: correlation with clinicopathological parameters. Histol Histopathol. 2007;22:305–9. doi: 10.14670/HH-22.305. [DOI] [PubMed] [Google Scholar]
- 30.Bergum C, Zoratti G, Boerner J, List K. Strong expression association between matriptase and its substrate prostasin in breast cancer. J Cell Physiol. 2012;227:1604–9. doi: 10.1002/jcp.22877. [DOI] [PubMed] [Google Scholar]
- 31.Zoratti GL, Tanabe LM, Hyland TE, Duhaime MJ, Colombo E, Leduc R, Marsault E, Johnson MD, Lin CY, Boerner J, Lang JE, List K. Matriptase regulates c-Met mediated proliferation and invasion in inflammatory breast cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidner KM, Arakaki N, Hartmann G, Vandekerckhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991;88:7001–5. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–3. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 34.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–42. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 35.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamed MM, Al-Raawi D, Sabet SF, El-Shinawi M. Inflammatory breast cancer: New factors contribute to disease etiology: A review. J Adv Res. 2014;5:525–36. doi: 10.1016/j.jare.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sales KU, Friis S, Konkel JE, Godiksen S, Hatakeyama M, Hansen KK, Rogatto SR, Szabo R, Vogel LK, Chen W, Gutkind JS, Bugge TH. Non-hematopoietic PAR-2 is essential for matriptase-driven pre-malignant progression and potentiation of ras-mediated squamous cell carcinogenesis. Oncogene. 2015;34:346–56. doi: 10.1038/onc.2013.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaffner F, Ruf W. Tissue factor and protease-activated receptor signaling in cancer. Semin Thromb Hemost. 2008;34:147–53. doi: 10.1055/s-2008-1079254. [DOI] [PubMed] [Google Scholar]
- 40.Camerer E, Barker A, Duong DN, Ganesan R, Kataoka H, Cornelissen I, Darragh MR, Hussain A, Zheng YW, Srinivasan Y, Brown C, Xu SM, Regard JB, Lin CY, Craik CS, Kirchhofer D, Coughlin SR. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev Cell. 2010;18:25–38. doi: 10.1016/j.devcel.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coughlin SR, Camerer E. PARticipation in inflammation. J Clin Invest. 2003;111:25–7. doi: 10.1172/JCI17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol. 2012;34:133–49. doi: 10.1007/s00281-011-0289-1. [DOI] [PubMed] [Google Scholar]
- 43.Santulli RJ, Derian CK, Darrow AL, Tomko KA, Eckardt AJ, Seiberg M, Scarborough RM, Andrade-Gordon P. Evidence for the presence of a protease-activated receptor distinct from the thrombin receptor in human keratinocytes. Proc Natl Acad Sci U S A. 1995;92:9151–5. doi: 10.1073/pnas.92.20.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou L, Kapas S, Cruchley AT, Macey MG, Harriott P, Chinni C, Stone SR, Howells GL. Immunolocalization of protease-activated receptor-2 in skin: receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology. 1998;94:356–62. doi: 10.1046/j.1365-2567.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 46.Bocheva G, Rattenholl A, Kempkes C, Goerge T, Lin CY, D'Andrea MR, Stander S, Steinhoff M. Role of matriptase and proteinase-activated receptor-2 in nonmelanoma skin cancer. J Invest Dermatol. 2009;129:1816–23. doi: 10.1038/jid.2008.449. [DOI] [PubMed] [Google Scholar]
- 47.Szabo R, Rasmussen AL, Moyer AB, Kosa P, Schafer JM, Molinolo AA, Gutkind JS, Bugge TH. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene. 2011;30:2003–16. doi: 10.1038/onc.2010.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhatt AS, Welm A, Farady CJ, Vasquez M, Wilson K, Craik CS. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci U S A. 2007;104:5771–6. doi: 10.1073/pnas.0606514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leonard EJ. Biological aspects of macrophage-stimulating protein (MSP) and its receptor. Ciba Found Symp. 1997;212:183–91. discussion 192-7. [PubMed] [Google Scholar]
- 50.Chen YQ, Zhou YQ, Fu LH, Wang D, Wang MH. Multiple pulmonary adenomas in the lung of transgenic mice overexpressing the RON receptor tyrosine kinase. Recepteur d'origine nantais. Carcinogenesis. 2002;23:1811–9. doi: 10.1093/carcin/23.11.1811. [DOI] [PubMed] [Google Scholar]
- 51.Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 2000;275:36720–5. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- 52.Forbs D, Thiel S, Stella MC, Sturzebecher A, Schweinitz A, Steinmetzer T, Sturzebecher J, Uhland K. In vitro inhibition of matriptase prevents invasive growth of cell lines of prostate and colon carcinoma. Int J Oncol. 2005;27:1061–70. [PubMed] [Google Scholar]
- 53.Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–42. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 54.Kilpatrick LM, Harris RL, Owen KA, Bass R, Ghorayeb C, Bar-Or A, Ellis V. Initiation of plasminogen activation on the surface of monocytes expressing the type II transmembrane serine protease matriptase. Blood. 2006;108:2616–23. doi: 10.1182/blood-2006-02-001073. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki M, Kobayashi H, Kanayama N, Saga Y, Suzuki M, Lin CY, Dickson RB, Terao T. Inhibition of tumor invasion by genomic down-regulation of matriptase through suppression of activation of receptor-bound pro-urokinase. J Biol Chem. 2004;279:14899–908. doi: 10.1074/jbc.M313130200. [DOI] [PubMed] [Google Scholar]
- 56.Scherl-Mostageer M, Sommergruber W, Abseher R, Hauptmann R, Ambros P, Schweifer N. Identification of a novel gene, CDCP1, overexpressed in human colorectal cancer. Oncogene. 2001;20:4402–8. doi: 10.1038/sj.onc.1204566. [DOI] [PubMed] [Google Scholar]
- 57.Bhatt AS, Erdjument-Bromage H, Tempst P, Craik CS, Moasser MM. Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene. 2005;24:5333–43. doi: 10.1038/sj.onc.1208582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He Y, Wortmann A, Burke LJ, Reid JC, Adams MN, Abdul-Jabbar I, Quigley JP, Leduc R, Kirchhofer D, Hooper JD. Proteolysis-induced N-terminal ectodomain shedding of the integral membrane glycoprotein CUB domain-containing protein 1 (CDCP1) is accompanied by tyrosine phosphorylation of its C-terminal domain and recruitment of Src and PKCdelta. J Biol Chem. 2010;285:26162–73. doi: 10.1074/jbc.M109.096453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ustach CV, Huang W, Conley-LaComb MK, Lin CY, Che M, Abrams J, Kim HR. A novel signaling axis of matriptase/PDGF-D/ss-PDGFR in human prostate cancer. Cancer Res. 2010;70:9631–40. doi: 10.1158/0008-5472.CAN-10-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lester BR, McCarthy JB. Tumor cell adhesion to the extracellular matrix and signal transduction mechanisms implicated in tumor cell motility, invasion and metastasis. Cancer Metastasis Rev. 1992;11:31–44. doi: 10.1007/BF00047601. [DOI] [PubMed] [Google Scholar]
- 62.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 63.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–9. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim Y, Stolarska MA, Othmer HG. The role of the microenvironment in tumor growth and invasion. Prog Biophys Mol Biol. 2011;106:353–79. doi: 10.1016/j.pbiomolbio.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ko CJ, Huang CC, Lin HY, Juan CP, Lan SW, Shyu HY, Wu SR, Hsiao PW, Huang HP, Shun CT, Lee MS. Androgen-Induced TMPRSS2 Activates Matriptase and Promotes Extracellular Matrix Degradation, Prostate Cancer Cell Invasion, Tumor Growth, and Metastasis. Cancer Res. 2015;75:2949–60. doi: 10.1158/0008-5472.CAN-14-3297. [DOI] [PubMed] [Google Scholar]
- 66.Satomi S, Yamasaki Y, Tsuzuki S, Hitomi Y, Iwanaga T, Fushiki T. A role for membrane-type serine protease (MT-SP1) in intestinal epithelial turnover. Biochem Biophys Res Commun. 2001;287:995–1002. doi: 10.1006/bbrc.2001.5686. [DOI] [PubMed] [Google Scholar]
- 67.Wu Q, Parry G. Hepsin and prostate cancer. Front Biosci. 2007;12:5052–9. doi: 10.2741/2447. [DOI] [PubMed] [Google Scholar]
- 68.Sardana G, Dowell B, Diamandis EP. Emerging biomarkers for the diagnosis and prognosis of prostate cancer. Clin Chem. 2008;54:1951–60. doi: 10.1373/clinchem.2008.110668. [DOI] [PubMed] [Google Scholar]
- 69.Pal P, Xi H, Guha S, Sun G, Helfand BT, Meeks JJ, Suarez BK, Catalona WJ, Deka R. Common variants in 8q24 are associated with risk for prostate cancer and tumor aggressiveness in men of European ancestry. Prostate. 2009;69:1548–56. doi: 10.1002/pros.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pal P, Xi H, Kaushal R, Sun G, Jin CH, Jin L, Suarez BK, Catalona WJ, Deka R. Variants in the HEPSIN gene are associated with prostate cancer in men of European origin. Hum Genet. 2006;120:187–92. doi: 10.1007/s00439-006-0204-3. [DOI] [PubMed] [Google Scholar]
- 71.Kim HJ, Han JH, Chang IH, Kim W, Myung SC. Variants in the HEPSIN gene are associated with susceptibility to prostate cancer. Prostate Cancer Prostatic Dis. 2012;15:353–8. doi: 10.1038/pcan.2012.17. [DOI] [PubMed] [Google Scholar]
- 72.Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, Dodd JG, Duckworth ML, Matusik RJ. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest. 1998;78:i–xv. [PubMed] [Google Scholar]
- 73.Klezovitch O, Chevillet J, Mirosevich J, Roberts RL, Matusik RJ, Vasioukhin V. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell. 2004;6:185–95. doi: 10.1016/j.ccr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 74.Tervonen TA, Belitskin D, Pant SM, Englund JI, Marques E, Ala-Hongisto H, Nevalaita L, Sihto H, Heikkila P, Leidenius M, Hewitson K, Ramachandra M, Moilanen A, Joensuu H, Kovanen PE, Poso A, Klefstrom J. Deregulated hepsin protease activity confers oncogenicity by concomitantly augmenting HGF/MET signalling and disrupting epithelial cohesion. Oncogene. 2016;35:1832–46. doi: 10.1038/onc.2015.248. [DOI] [PubMed] [Google Scholar]
- 75.Miao J, Mu D, Ergel B, Singavarapu R, Duan Z, Powers S, Oliva E, Orsulic S. Hepsin colocalizes with desmosomes and induces progression of ovarian cancer in a mouse model. Int J Cancer. 2008;123:2041–7. doi: 10.1002/ijc.23726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xing P, Li JG, Jin F, Zhao TT, Liu Q, Dong HT, Wei XL. Clinical and biological significance of hepsin overexpression in breast cancer. J Investig Med. 2011;59:803–10. doi: 10.2310/JIM.0b013e31821451a1. [DOI] [PubMed] [Google Scholar]
- 77.Herter S, Piper DE, Aaron W, Gabriele T, Cutler G, Cao P, Bhatt AS, Choe Y, Craik CS, Walker N, Meininger D, Hoey T, Austin RJ. Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane-anchored serine protease implicated in prostate and ovarian cancers. Biochem J. 2005;390:125–36. doi: 10.1042/BJ20041955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ganesan R, Kolumam GA, Lin SJ, Xie MH, Santell L, Wu TD, Lazarus RA, Chaudhuri A, Kirchhofer D. Proteolytic activation of pro-macrophage-stimulating protein by hepsin. Mol Cancer Res. 2011;9:1175–86. doi: 10.1158/1541-7786.MCR-11-0004. [DOI] [PubMed] [Google Scholar]
- 79.Kirchhofer D, Peek M, Lipari MT, Billeci K, Fan B, Moran P. Hepsin activates pro-hepatocyte growth factor and is inhibited by hepatocyte growth factor activator inhibitor-1B (HAI-1.B) and HAI-2. FEBS Lett. 2005;579:1945–50. doi: 10.1016/j.febslet.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 80.Moran P, Li W, Fan B, Vij R, Eigenbrot C, Kirchhofer D. Pro-urokinase-type plasminogen activator is a substrate for hepsin. J Biol Chem. 2006;281:30439–46. doi: 10.1074/jbc.M605440200. [DOI] [PubMed] [Google Scholar]
- 81.Chen M, Chen LM, Lin CY, Chai KX. Hepsin activates prostasin and cleaves the extracellular domain of the epidermal growth factor receptor. Mol Cell Biochem. 2010;337:259–66. doi: 10.1007/s11010-009-0307-y. [DOI] [PubMed] [Google Scholar]
- 82.Friis S, Uzzun Sales K, Godiksen S, Peters DE, Lin CY, Vogel LK, Bugge TH. A matriptase-prostasin reciprocal zymogen activation complex with unique features: prostasin as a non-enzymatic co-factor for matriptase activation. J Biol Chem. 2013;288:19028–39. doi: 10.1074/jbc.M113.469932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.List K, Hobson JP, Molinolo A, Bugge TH. Co-localization of the channel activating protease prostasin/(CAP1/PRSS8) with its candidate activator, matriptase. J Cell Physiol. 2007;213:237–45. doi: 10.1002/jcp.21115. [DOI] [PubMed] [Google Scholar]
- 84.Partanen JI, Tervonen TA, Myllynen M, Lind E, Imai M, Katajisto P, Dijkgraaf GJ, Kovanen PE, Makela TP, Werb Z, Klefstrom J. Tumor suppressor function of Liver kinase B1 (Lkb1) is linked to regulation of epithelial integrity. Proc Natl Acad Sci U S A. 2012;109:E388–97. doi: 10.1073/pnas.1120421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol. 1996;148:313–9. [PMC free article] [PubMed] [Google Scholar]
- 86.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr., Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–86. [PubMed] [Google Scholar]
- 87.Jacquinet E, Rao NV, Rao GV, Zhengming W, Albertine KH, Hoidal JR. Cloning and characterization of the cDNA and gene for human epitheliasin. Eur J Biochem. 2001;268:2687–99. doi: 10.1046/j.1432-1327.2001.02165.x. [DOI] [PubMed] [Google Scholar]
- 88.Vaarala MH, Porvari K, Kyllonen A, Lukkarinen O, Vihko P. The TMPRSS2 gene encoding transmembrane serine protease is overexpressed in a majority of prostate cancer patients: detection of mutated TMPRSS2 form in a case of aggressive disease. Int J Cancer. 2001;94:705–10. doi: 10.1002/ijc.1526. [DOI] [PubMed] [Google Scholar]
- 89.Soller MJ, Isaksson M, Elfving P, Soller W, Lundgren R, Panagopoulos I. Confirmation of the high frequency of the TMPRSS2/ERG fusion gene in prostate cancer. Genes Chromosomes Cancer. 2006;45:717–9. doi: 10.1002/gcc.20329. [DOI] [PubMed] [Google Scholar]
- 90.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 91.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, Yu J, Wang L, Montie JE, Rubin MA, Pienta KJ, Roulston D, Shah RB, Varambally S, Mehra R, Chinnaiyan AM. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–9. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 92.Khemlina G, Ikeda S, Kurzrock R. Molecular landscape of prostate cancer: implications for current clinical trials. Cancer Treat Rev. 2015;41:761–6. doi: 10.1016/j.ctrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, Hoshida Y, Mosquera JM, Pawitan Y, Lee C, Adami HO, Mucci LA, Kantoff PW, Andersson SO, Chinnaiyan AM, Johansson JE, Rubin MA. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–9. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 94.Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD, Hood L, Nelson PS. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4. [PubMed] [Google Scholar]
- 95.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, True LD, Morrissey C, Corey E, Montgomery B, Mostaghel E, Clegg N, Coleman I, Brown CM, Schneider EL, Craik C, Simon JA, Bedalov A, Nelson PS. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–25. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson S, Greer B, Hooper J, Zijlstra A, Walker B, Quigley J, Hawthorne S. The membrane-anchored serine protease, TMPRSS2, activates PAR-2 in prostate cancer cells. Biochem J. 2005;388:967–72. doi: 10.1042/BJ20041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wallrapp C, Hahnel S, Muller-Pillasch F, Burghardt B, Iwamura T, Ruthenburger M, Lerch MM, Adler G, Gress TM. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 2000;60:2602–6. [PubMed] [Google Scholar]
- 100.de Aberasturi AL, Calvo A. TMPRSS4: an emerging potential therapeutic target in cancer. Br J Cancer. 2015;112:4–8. doi: 10.1038/bjc.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohler A, Becker-Pauly C. TMPRSS4 is a type II transmembrane serine protease involved in cancer and viral infections. Biol Chem. 2012;393:907–14. doi: 10.1515/hsz-2012-0155. [DOI] [PubMed] [Google Scholar]
- 102.Guan H, Liang W, Liu J, Wei G, Li H, Xiu L, Xiao H, Li Y. Transmembrane protease serine 4 promotes thyroid cancer proliferation via CREB phosphorylation. Thyroid. 2015;25:85–94. doi: 10.1089/thy.2014.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jung H, Lee KP, Park SJ, Park JH, Jang YS, Choi SY, Jung JG, Jo K, Park DY, Yoon JH, Park JH, Lim DS, Hong GR, Choi C, Park YK, Lee JW, Hong HJ, Kim S, Park YW. TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene. 2008;27:2635–47. doi: 10.1038/sj.onc.1210914. [DOI] [PubMed] [Google Scholar]
- 104.Larzabal L, Nguewa PA, Pio R, Blanco D, Sanchez B, Rodriguez MJ, Pajares MJ, Catena R, Montuenga LM, Calvo A. Overexpression of TMPRSS4 in non-small cell lung cancer is associated with poor prognosis in patients with squamous histology. Br J Cancer. 2011;105:1608–14. doi: 10.1038/bjc.2011.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee Y, Ko D, Min HJ, Kim SB, Ahn HM, Lee Y, Kim S. TMPRSS4 induces invasion and proliferation of prostate cancer cells through induction of Slug and cyclin D1. Oncotarget. 2016 doi: 10.18632/oncotarget.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim S, Kang HY, Nam EH, Choi MS, Zhao XF, Hong CS, Lee JW, Lee JH, Park YK. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis. 2010;31:597–606. doi: 10.1093/carcin/bgq024. [DOI] [PubMed] [Google Scholar]
- 107.Larzabal L, de Aberasturi AL, Redrado M, Rueda P, Rodriguez MJ, Bodegas ME, Montuenga LM, Calvo A. TMPRSS4 regulates levels of integrin alpha5 in NSCLC through miR-205 activity to promote metastasis. Br J Cancer. 2014;110:764–74. doi: 10.1038/bjc.2013.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keppner A, Andreasen D, Merillat AM, Bapst J, Ansermet C, Wang Q, Maillard M, Malsure S, Nobile A, Hummler E. Epithelial Sodium Channel-Mediated Sodium Transport Is Not Dependent on the Membrane-Bound Serine Protease CAP2/Tmprss4. PLoS One. 2015;10:e0135224. doi: 10.1371/journal.pone.0135224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Enyedy IJ, Lee SL, Kuo AH, Dickson RB, Lin CY, Wang S. Structure-based approach for the discovery of bis-benzamidines as novel inhibitors of matriptase. J Med Chem. 2001;44:1349–55. doi: 10.1021/jm000395x. [DOI] [PubMed] [Google Scholar]
- 110.Long YQ, Lee SL, Lin CY, Enyedy IJ, Wang S, Li P, Dickson RB, Roller PP. Synthesis and evaluation of the sunflower derived trypsin inhibitor as a potent inhibitor of the type II transmembrane serine protease, matriptase. Bioorg Med Chem Lett. 2001;11:2515–9. doi: 10.1016/s0960-894x(01)00493-0. [DOI] [PubMed] [Google Scholar]
- 111.Sun J, Pons J, Craik CS. Potent and selective inhibition of membrane-type serine protease 1 by human single-chain antibodies. Biochemistry. 2003;42:892–900. doi: 10.1021/bi026878f. [DOI] [PubMed] [Google Scholar]
- 112.Galkin AV, Mullen L, Fox WD, Brown J, Duncan D, Moreno O, Madison EL, Agus DB. CVS-3983, a selective matriptase inhibitor, suppresses the growth of androgen independent prostate tumor xenografts. Prostate. 2004;61:228–35. doi: 10.1002/pros.20094. [DOI] [PubMed] [Google Scholar]
- 113.Desilets A, Longpre JM, Beaulieu ME, Leduc R. Inhibition of human matriptase by eglin c variants. FEBS Lett. 2006;580:2227–32. doi: 10.1016/j.febslet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 114.Quimbar P, Malik U, Sommerhoff CP, Kaas Q, Chan LY, Huang YH, Grundhuber M, Dunse K, Craik DJ, Anderson MA, Daly NL. High-affinity cyclic peptide matriptase inhibitors. J Biol Chem. 2013;288:13885–96. doi: 10.1074/jbc.M113.460030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gray K, Elghadban S, Thongyoo P, Owen KA, Szabo R, Bugge TH, Tate EW, Leatherbarrow RJ, Ellis V. Potent and specific inhibition of the biological activity of the type-II transmembrane serine protease matriptase by the cyclic microprotein MCoTI-II. Thromb Haemost. 2014;112:402–11. doi: 10.1160/TH13-11-0895. [DOI] [PubMed] [Google Scholar]
- 116.Colombo E, Desilets A, Duchene D, Chagnon F, Najmanovich R, Leduc R, Marsault E. Design and synthesis of potent, selective inhibitors of matriptase. ACS Med Chem Lett. 2012;3:530–4. doi: 10.1021/ml3000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han Z, Harris PK, Jones DE, Chugani R, Kim T, Agarwal M, Shen W, Wildman SA, Janetka JW. Inhibitors of HGFA, Matriptase, and Hepsin Serine Proteases: A Nonkinase Strategy to Block Cell Signaling in Cancer. ACS Med Chem Lett. 2014;5:1219–24. doi: 10.1021/ml500254r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Han Z, Harris PK, Karmakar P, Kim T, Owusu BY, Wildman SA, Klampfer L, Janetka JW. alpha-Ketobenzothiazole Serine Protease Inhibitors of Aberrant HGF/c-MET and MSP/RON Kinase Pathway Signaling in Cancer. ChemMedChem. 2016;11:585–99. doi: 10.1002/cmdc.201500600. [DOI] [PubMed] [Google Scholar]
- 119.Owusu BY, Bansal N, Venukadasula PK, Ross LJ, Messick TE, Goel S, Galemmo RA, Klampfer L. Inhibition of pro-HGF activation by SRI31215, a novel approach to block oncogenic HGF/MET signaling. Oncotarget. 2016 doi: 10.18632/oncotarget.8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, Szabo R, Fasano A, Bugge TH, Antalis TM. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A. 2010;107:4200–5. doi: 10.1073/pnas.0903923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.List K, Currie B, Scharschmidt TC, Szabo R, Shireman J, Molinolo A, Cravatt BF, Segre J, Bugge TH. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem. 2007;282:36714–23. doi: 10.1074/jbc.M705521200. [DOI] [PubMed] [Google Scholar]
- 122.Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben Amitai D, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, Magal N, Sprecher E, Shohat M. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet. 2007;80:467–77. doi: 10.1086/512487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 124.Franco FM, Jones DE, Harris PK, Han Z, Wildman SA, Jarvis CM, Janetka JW. Structure-based discovery of small molecule hepsin and HGFA protease inhibitors: Evaluation of potency and selectivity derived from distinct binding pockets. Bioorg Med Chem. 2015;23:2328–43. doi: 10.1016/j.bmc.2015.03.072. [DOI] [PubMed] [Google Scholar]
- 125.Tang X, Mahajan SS, Nguyen LT, Beliveau F, Leduc R, Simon JA, Vasioukhin V. Targeted inhibition of cell-surface serine protease Hepsin blocks prostate cancer bone metastasis. Oncotarget. 2014;5:1352–62. doi: 10.18632/oncotarget.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li W, Wang BE, Moran P, Lipari T, Ganesan R, Corpuz R, Ludlam MJ, Gogineni A, Koeppen H, Bunting S, Gao WQ, Kirchhofer D. Pegylated kunitz domain inhibitor suppresses hepsin-mediated invasive tumor growth and metastasis. Cancer Res. 2009;69:8395–402. doi: 10.1158/0008-5472.CAN-09-1995. [DOI] [PubMed] [Google Scholar]
- 127.Koschubs T, Dengl S, Durr H, Kaluza K, Georges G, Hartl C, Jennewein S, Lanzendorfer M, Auer J, Stern A, Huang KS, Packman K, Gubler U, Kostrewa D, Ries S, Hansen S, Kohnert U, Cramer P, Mundigl O. Allosteric antibody inhibition of human hepsin protease. Biochem J. 2012;442:483–94. doi: 10.1042/BJ20111317. [DOI] [PubMed] [Google Scholar]
- 128.Xuan JA, Schneider D, Toy P, Lin R, Newton A, Zhu Y, Finster S, Vogel D, Mintzer B, Dinter H, Light D, Parry R, Polokoff M, Whitlow M, Wu Q, Parry G. Antibodies neutralizing hepsin protease activity do not impact cell growth but inhibit invasion of prostate and ovarian tumor cells in culture. Cancer Res. 2006;66:3611–9. doi: 10.1158/0008-5472.CAN-05-2983. [DOI] [PubMed] [Google Scholar]
- 129.Heinemann V, Ebert MP, Laubender RP, Bevan P, Mala C, Boeck S. Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. Br J Cancer. 2013;108:766–70. doi: 10.1038/bjc.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu Q, Yu D, Post J, Halks-Miller M, Sadler JE, Morser J. Generation and characterization of mice deficient in hepsin, a hepatic transmembrane serine protease. J Clin Invest. 1998;101:321–6. doi: 10.1172/JCI1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu IS, Chen HJ, Lee YS, Huang PH, Lin SR, Tsai TW, Lin SW. Mice deficient in hepsin, a serine protease, exhibit normal embryogenesis and unchanged hepatocyte regeneration ability. Thromb Haemost. 2000;84:865–70. [PubMed] [Google Scholar]
- 132.Guipponi M, Tan J, Cannon PZ, Donley L, Crewther P, Clarke M, Wu Q, Shepherd RK, Scott HS. Mice deficient for the type II transmembrane serine protease, TMPRSS1/hepsin, exhibit profound hearing loss. Am J Pathol. 2007;171:608–16. doi: 10.2353/ajpath.2007.070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hsu YC, Huang HP, Yu IS, Su KY, Lin SR, Lin WC, Wu HL, Shi GY, Tao MH, Kao CH, Wu YM, Martin PE, Lin SY, Yang PC, Lin SW. Serine protease hepsin regulates hepatocyte size and hemodynamic retention of tumor cells by hepatocyte growth factor signaling in mice. Hepatology. 2012;56:1913–23. doi: 10.1002/hep.25773. [DOI] [PubMed] [Google Scholar]
- 134.Meyer D, Sielaff F, Hammami M, Bottcher-Friebertshauser E, Garten W, Steinmetzer T. Identification of the first synthetic inhibitors of the type II transmembrane serine protease TMPRSS2 suitable for inhibition of influenza virus activation. Biochem J. 2013;452:331–43. doi: 10.1042/BJ20130101. [DOI] [PubMed] [Google Scholar]
- 135.Garten W, Braden C, Arendt A, Peitsch C, Baron J, Lu Y, Pawletko K, Hardes K, Steinmetzer T, Bottcher-Friebertshauser E. Influenza virus activating host proteases: Identification, localization and inhibitors as potential therapeutics. Eur J Cell Biol. 2015;94:375–83. doi: 10.1016/j.ejcb.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 136.Kim TS, Heinlein C, Hackman RC, Nelson PS. Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Mol Cell Biol. 2006;26:965–75. doi: 10.1128/MCB.26.3.965-975.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kang S, Min HJ, Kang MS, Jung MG, Kim S. Discovery of novel 2-hydroxydiarylamide derivatives as TMPRSS4 inhibitors. Bioorg Med Chem Lett. 2013;23:1748–51. doi: 10.1016/j.bmcl.2013.01.055. [DOI] [PubMed] [Google Scholar]