Abstract
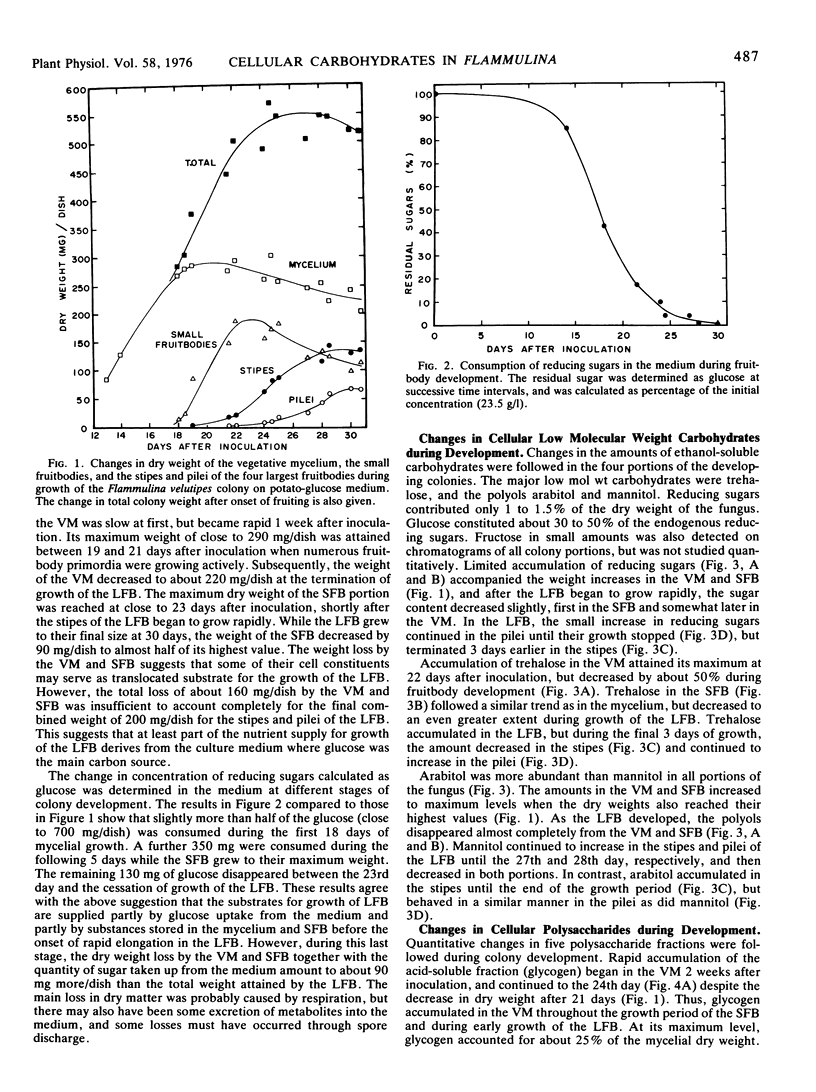
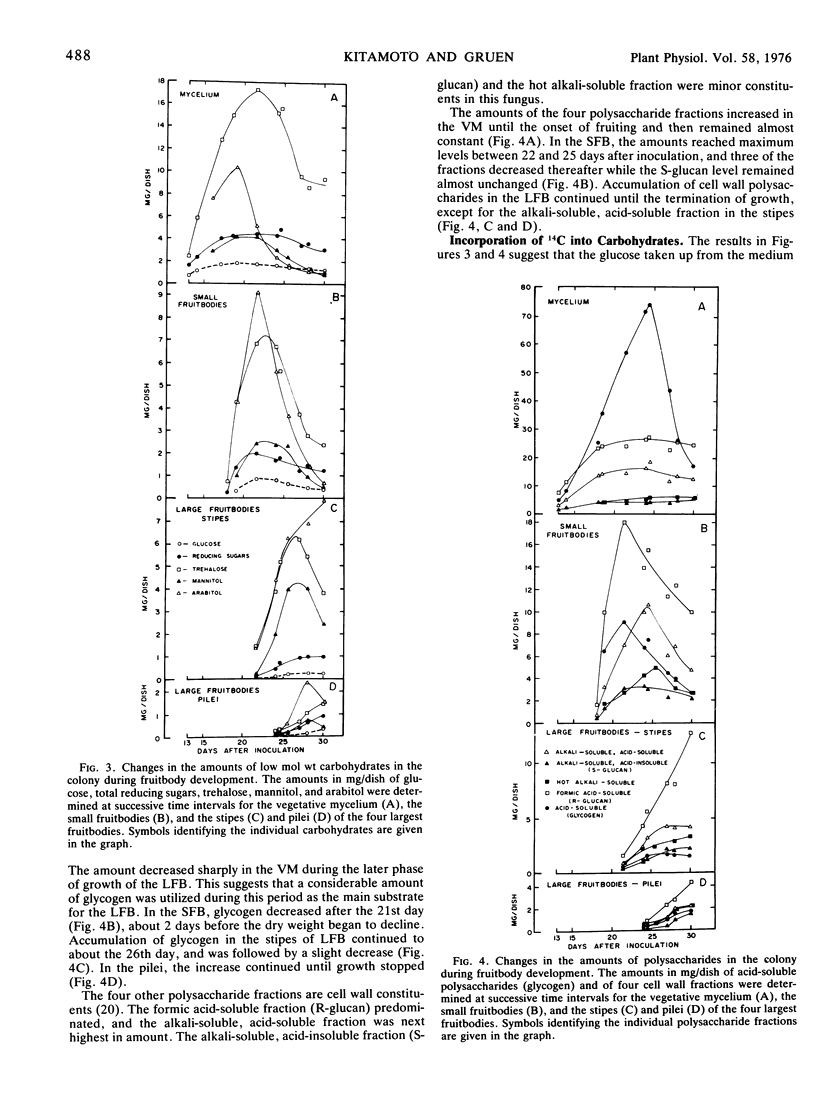
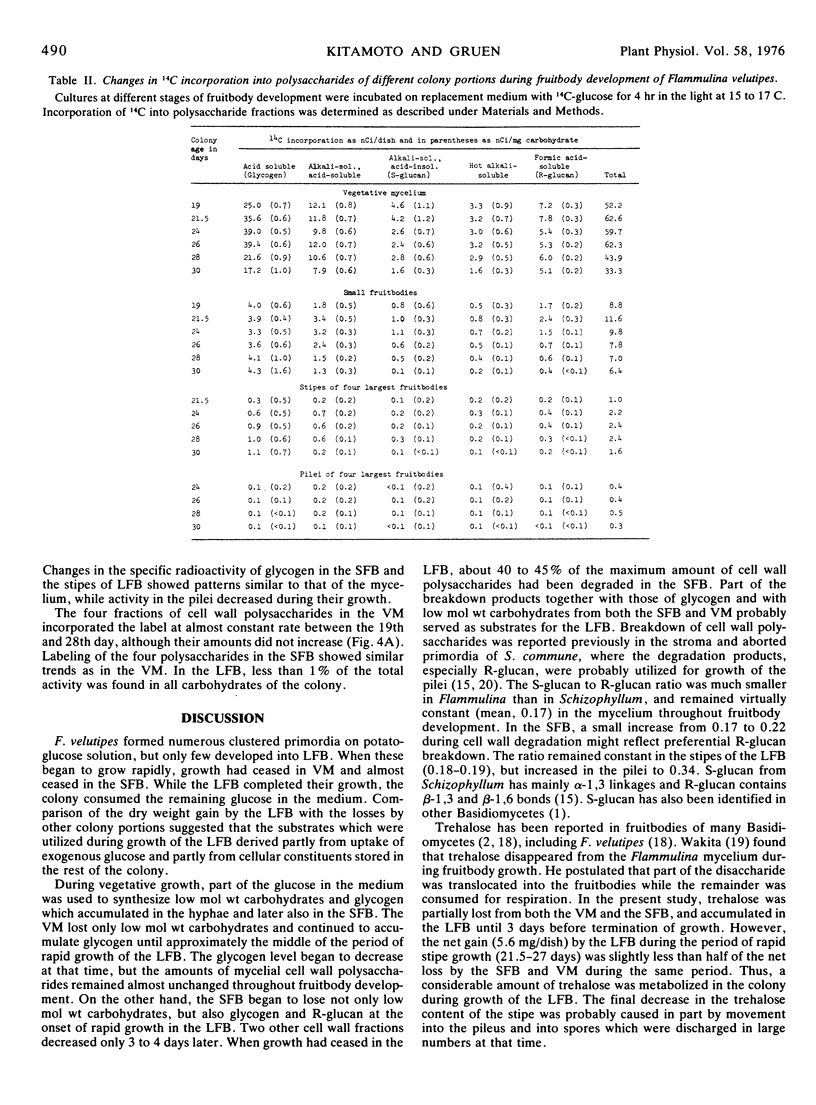
Flammulina velutipes (Curt. ex Fr.) Sing. was grown on potato-glucose solution freed of most starch. Glucose uptake and dry weight changes in the colony indicated that the large fruitbodies derived their substrates partly from glucose remaining in the medium and partly from cellular constituents stored in the mycelium and small fruitbodies. Changes in the amounts of low molecular weight carbohydrates, glycogen, and four cell wall polysaccharide fractions were followed in the mycelium and fruitbodies. Trehalose, arabitol, and smaller amounts of mannitol were the main stored low molecular weight carbohydrates. A large net loss of these compounds occurred in the mycelium and small fruitbodies after their growth ceased. The carbohydrates accumulated in the large fruitbodies, but were also partly metabolized in the colony. Reducing sugars were minor components, and included about 30 to 50% glucose and a small undetermined quantity of fructose. Glycogen was the main storage carbohydrate in the mycelium, and was also stored in the small fruitbodies. It was broken down in both structures during growth of the large fruitbodies which accumulated only small amounts. During the same period, almost 45% of the maximum amount of cell wall polysaccharides were degraded in the small fruitbodies, but not in the mycelium.
By feeding 14C-glucose in replacement medium, incorporation of radioactivity into carbohydrates was followed in the colony during fruit-body development. Total incorporation was highest in trehalose, next highest in glycogen, and the rest was found in polyols and cell wall polysaccharides except for a few per cent which remained in endogenous glucose. In the large fruitbodies, specific radioactivity in glucose was much lower than in trehalose and mannitol. The labeling patterns in the mycelium and large fruitbodies suggested that trehalose, mannitol, and possibly arabitol were translocated into the stipes and pilei.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon J. S., Jones D., Farmer V. C., Webley D. M. The occurrence of alpha(1-3)glucan in Cryptococcus, Schizosaccharomyces and Polyporus species, and its hydrolysis by a Streptomyces culture filtrate lysing cell walls of Cryptococcus. Biochim Biophys Acta. 1968 May;158(2):313–315. doi: 10.1016/0304-4165(68)90153-0. [DOI] [PubMed] [Google Scholar]
- Cotter D. A., Niederpruem D. J. Control of arabitol formation in Schizophyllum commune development. Arch Mikrobiol. 1971;78(2):128–138. doi: 10.1007/BF00424869. [DOI] [PubMed] [Google Scholar]
- Dütsch G., Rast D. Biosynthese von Mannitol in Agaricus bisporus. Arch Mikrobiol. 1969;65(3):195–207. doi: 10.1007/BF00407103. [DOI] [PubMed] [Google Scholar]
- Niederpruem D. J., Wessels J. G. Cytodifferentiation and morphogenesis in Schizophyllum commune. Bacteriol Rev. 1969 Dec;33(4):505–535. doi: 10.1128/br.33.4.505-535.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES W. R., REYNOLDS T. A solvent for the paper chromatographic separation of glucose and sorbitol. Nature. 1958 Mar 15;181(4611):767–768. doi: 10.1038/181767a0. [DOI] [PubMed] [Google Scholar]


