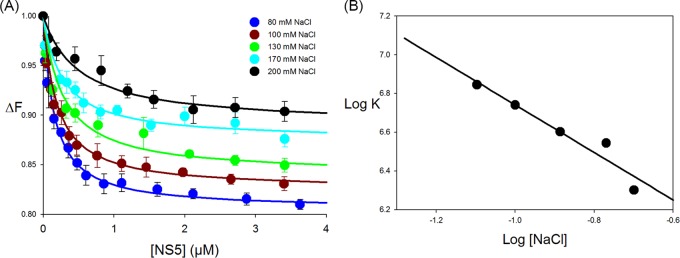
FIG 3.
Effect of salt on SLA80-NS5 binding. (A) Fluorescence titrations of fluorescein-labeled SLA80 with NS5 (λex = 480 nm; λem = 520 nm) in buffer B1 (50 mM Tris [pH 7.5] at 10°C, 1 mM MgCl2) containing the following different NaCl concentrations: 80 mM, 100 mM, 130 mM, 170 mM, and 200 mM. The concentration of fluorescein-labeled SLA80 is 1.5 × 10−8 M. Increasing salt concentrations decrease the affinity of the polymerase for SLA80. The solid lines are nonlinear least-squares fits of the titration curve with a K of 7.0 × 106 M−1 (80 mM NaCl), a K of 5.5 × 106 M−1 (100 mM NaCl), a K of 4.0 × 106 M−1 (130 mM NaCl), a K of 3.5 × 106 M−1 (170 mM NaCl), and a K of 2.0 × 106 M−1 (200 mM NaCl). (B) Dependence of the logarithm of the intrinsic binding constant, K, on the logarithm of NaCl concentrations. The solid line is the linear least-squares fit of the NaCl concentration regions of the plot, which provide the slope, ∂logK/∂log[NaCl] = −1.3.