Abstract
Background
The SnRKs (sucrose non-fermenting 1 related protein kinase) are a gene family coding for Ser/Thr protein kinases and play important roles in linking the tolerance and metabolic responses of plants to abiotic stresses. To date, no genome-wide characterization of the sucrose non-ferment 1 related protein kinase 2 (SnRK2) subfamily has been conducted in potato (Solanum tuberosum L.).
Results
In this study, eight StSnRK2 genes (StSnRK2.1- StSnRK2.8) were identified in the genome of the potato (Solanum tuberosum L.) cultivar ‘Longshu 3’, with similar characteristics to SnRK2 from other plant species in gene structure, motif distribution and secondary structures. The C-terminal regions were highly divergent among StSnRK2s, while they all carried the similar Ser/Thr protein kinase domain. The fluorescence of GFP fused with StSnRK2.1, StSnRK2.2, StSnRK2.6, StSnRK2.7 and StSnRK2.8 was detected in the nucleus and cytoplasm of onion epidermal cells with StSnRK2.3 and StSnRK2.4 mainly associated to the nucleus while StSnRK2.5 to subcellular organelles. Expression level analysis by qRT-PCR showed that StSnRK2.1, 2.2, 2.5 and 2.6 were more than 1 fold higher in the root than in the leaf, tuber and stem tissues. The expressions of StSnRK2.3, 2.7, and 2.8 were at least 1.5 folds higher in the leaf and stem than in the root, but lower in the tuber. The expression of StSnRK2.4 was also significantly (P < 0.05) higher in leaf, stem, and tuber than in the root. From the perspective of the relative expressions of StSnRK2 genes in potato, ABA treatment had a different effect from NaCl and PEG treatments.
Conclusion
In the present study, we identified and characterized eight SnRK2s in the potato genome. The eight StSnRK2s exhibit similar gene structure and secondary structures in potato to the SnRK2s found in other plant species. The relative expression of eight genes varied among various tissues (roots, leaves, tubers, and stems) and abiotic stresses (ABA, NaCl and PEG-6000) with the prolongation of treatments. This study provides valuable information for the future functional dissection of potato SnRK2 genes in stress signal transduction, plant growth and development.
Electronic supplementary material
The online version of this article (doi:10.1186/s12863-017-0506-6) contains supplementary material, which is available to authorized users.
Keywords: Potato, StSnRK2, Stress responses , ABA, NaCl, PEG-6000
Background
In many regions of the world, potato (Solanum tuberosum L.) production is seriously threatened by abiotic stresses such as frequent drought and salinity [1]. Plant drought tolerance is a complex response and involves the comprehensive interactions of numerous genes, proteins and metabolites in plant cells. Systematic analysis of the plant cell network responsible for drought stress tolerance is a promising approach for the development of drought tolerant plants [1, 2]. Protein phosphorylation is involved in regulation of various cellular activities in plants and one of the main signals mediating the responses to environmental stresses [3–7]. The SnRKs (sucrose non-fermenting 1 related protein kinase) are a gene family coding for Ser/Thr protein kinases and play important roles in linking abiotic stress tolerance and the metabolic responses of plants [8–10]. Based on sequence similarity, domain structure and metabolic roles, the plant SnRK family is divided into three subfamilies: SnRK1, SnRK2 and SnRK3. Many studies have demonstrated that these three subfamilies play various roles in the metabolism and development of plants. SnRK1 plays an important role in regulating carbon metabolism and energy conversion in plants [11, 12], while SnRK3 is involved in plant development, calcium-responsive regulatory loop and abscisic acid (ABA) sensitivity. SnRK2 members are the major players in plant responses to osmotic stresses [13–16], ABA dependent and independent stomatal closure-opening [17], fruit development [18], seed dormancy [19] and germination [20, 21].
Since the first SnRK2 (PKABA1) gene was identified in wheat [22], the members of the SnRK2 subfamily have been subsequently identified in many other plant species such as Arabidopsis [23, 24], rice [25], maize [26], tobacco [27], wheat [28, 29], sorghum [30], soybean [31, 32], barley [33] and grape [34]. In Arabidopsis, nine of the ten SnRK2 members (except for SnRK2.9) can be activated by osmotic stress [13]. Among them, SnRK2.8 and SnRK2.7 play a critical role in regulating the expression of drought-responsive genes [35]. In rice, all the ten SnRK2 members (designated as SAPK1 to SAPK10, stress/ABA-activated protein kinase) are activated by hyperosmotic stress with three of them (SAPK8, SAPK9, and SAPK10) also activated by ABA [25, 36]. In maize, all the ten characterized members of SnRK2 are stress-related [26]. In wheat, osmotic stress and ABA-induced gene expression were linked with the activity of SnRK2 members [28, 29]. However, little is known about the functions of SnRK2s in potato and there is no information on how SnRK2 family genes are involved in the tolerance of potato to osmotic stress.
In this study, we identified and characterized eight SnRK2 genes from the potato genome (named StSnRK2.1, StSnRK2.2, StSnRK2.3, StSnRK2.4, StSnRK2.5, StSnRK2.6, StSnRK2.7 and StSnRK2.8), then analyzed their tissue-specific and stress-induced expression profiles. To better understand their mechanisms underlying improved abiotic stress tolerance of the StSnRK2 gene family, the subcellular localization of StSnRK2 proteins, the expression patterns of the eight SnRK2s gene members and physiological index analysis of potato plantlets responding to ABA (50 μM), NaCl (200 mM) and PEG-6000 (5%) were performed. This study established functions for the potato SnRK2 gene family and provides a foundation for further clarifying the mechanism of potato stress resistance. The results will also provide genetic foundations for developing drought tolerant potato cultivars via manipulating SnRK2 gene family.
Methods
Plant material
One the local main potato cultivars, ‘Longshu 3’ released by Gansu Academy of Agricultural Sciences, Lanzhou, China, was used in the study. This cultivar is widely grown in northwestern China because of its moderate resistance to low temperatures, drought and salinity. Potato plantlets were propagated in MS medium [37]. A total of 6–8 plantlets were cultured in each 150 ml flask at an illumination intensity of 200 μmol m2 s−1 under the temperature of 23 ± 2 °C with a photoperiod of 16 h/8 h (day/night).
Identification and cloning of potato StSnRK2s
The coding sequences of SnRK2s from Arabidopsis, maize, and rice were obtained through literature and the genbank (http://www.ncbi.nlm.nih.gov) (Additional file 1: Table S1). To identify the SnRK2 genes in potato, we looked for the highly conserved sequences of SnRK2s in the Potato Genome Sequencing Consortium database (http://potatogenome.net/index.php/Main_Page).
To clone the cDNA sequences, total RNA was extracted from the potato plantlets using the RNA simple Total RNA Kit (TIANGEN). The quality and quantity of RNA were measured by both electrophoresis and optical absorbency (Nanophotometer, Implen). The cDNA was synthesized from the total RNA following the instruction of the Kit cDNA MMLV (Sangon). Full-length coding sequences of StSnRK2 genes were amplified via PCR with the primers presented in Additional file 1: Table S2. The PCR products were inserted into pGEM-T easy cloning vector and propagated in Escherichia coli DH5α (Promega) for sequencing and conservation.
Phylogenetic analysis
The SnRK2s from Arabidopsis, rice, maize and potato were aligned using ClustalX1.81 (ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX). The final view of the alignment was manually adjusted with Jalview (2.07) [38]. The phylogenetic tree was constructed using the neighbor-joining (NJ) method in MEGA 5 [39]. Bootstrap analysis was performed using 1000 replicates to evaluate the reliability of different phylogenetic groups.
Characterization of StSnRK2 genes and proteins
The genomic sequences were identified from Potato Genome Sequencing Consortium database (http://potatogenome.net/index.php/Main_Page). The gene structures (exon and intron) were analyzed with splign (http://www.ncbi.nlm.nih.gov/sutils/splign) [40]. The promoter sequences were identified about 2 kb upstream of the transcription start site of each gene, and the abiotic stress-associated elements (ABRE, DRE/CRT, and LTRE) were obtained from the PLACE database (http://www.dna.affrc.go.jp/) [41]. The secondary structure of the deduced polypeptide was predicted using the programs of SOPMA which listed in Expasy (www.EXPASY.org)
Subcellular localization of StSnRK2s
To determine the subcellular localization of the StSnRK2s, full-length of StSnRK2s were inserted into the KpnI/BamHI site of the pEBGFP vector to generate pEBGFP-StSnRK2. For transient expression in onion epidermal cells, the pEBGFP and pEBGFP-StSnRK2s plasmids and the constructs were introduced into onion epidermal cells using Agrobacterium tumefaciens-mediated transformation methods. Onion epidermal tissues were subsequently incubated on solid MS medium in the dark at 26 °C for 48 h. Localization of fluorescent proteins in onion epidermal cells was observed by a Zeiss LSM 710/ConfoCor2 laser-scanning imaging system (CarlZeiss, Jena, Germany). Fluorescence was detected between 505 and 550 nm with excitation at 488 nm. GFP fluorescence and light field vision were recorded in separate channels and then merged into an overlay image.
Expression profile of StSnRK2s in potato plants
Four-week old potato in vitro plantlets were transferred into liquid MS medium supplemented with either 50 μM ABA, 200 mM sodium chloride (NaCl) or 5% polyethyleneglycol (PEG-6000) to investigate the expression patterns of StSnRK2s under different stress-conditions. After incubation 24 h, the leaves were harvested and immediately frozen in liquid nitrogen for RNA extraction. Quantitative Real-Time PCR (qRT-PCR) was conducted with SYBR Green I technology (TaKaRa), the ef1α gene (AB061263) was used as internal control gene, the gene primers presented in Additional file 1: Table S2 and the relative quantification of RNA expression was selected. Three independent replicates were performed for each experiment. In each case, ten test tubes containing six to eight explants represented each treatment. For quantification, a no-template control, calibration curve and non-specific reactions were run in triplicate. The 2-△△Ct method (Ct, Cycle threshold value of target gene) was selected to calculate the gene expression folds; The formulas are as follows [42, 43]:
where control gene refers to those genes that were treated with only water.
Gene expression fold (Test gene/Control gene) = 2− △ △Ct.
Total soluble sugar and proline content assay
In vitro plantlets treated in the same manner as for gene expression analysis were sampled for total soluble sugar and proline content assay under different stress conditions. The total soluble sugar content in the leaves was measured by the method of Zhang et al. [44]. The total soluble sugar was extracted from 100 mg potato leaves in boiling water and the supernatant was mixed with 80% ethanol to a final volume of 25 ml. Then, 2 mL of solution was mixed with 5 mL of anthrone reagent and the mixture was incubated at room temperature (18–30 °C) for 15 min. The absorbance was at 630 nm (UV-2600, Shomadzu) and the sugar concentration was determined from a glucose standard curve and expressed on a fresh weight base.
Proline content was assayed by the acid-ninhydrin procedure [45]. Potato leaf samples (0.5 g) were ground with 3% sulphosalicylic acid (10 mL). After centrifugation at 5000 rpm for 10 min, supernatant (2 mL) was mixed with the same volume of acidninhydrin and acetic acid, the mixture was incubated at 100 °C for 1 h, and the reaction was stopped on ice. The mixture was extracted with 4 mL toluene and absorbance at 517 nm was determined (UV-2600, Shimadzu). The proline concentration was determined from a standard curve and expressed on a fresh weight base.
Statistical analysis
Gene expression, total soluble sugar, and proline assays were performed with three replicates and were analyzed using the Statistical Package for Social Sciences for Windows (SPSS version 17.0, SPSS Inc., Chicago, USA). Mean comparisons were performed using Tukey’s multiple range test and the significance between different treatments was presented at the P < 0.05 probability level.
Results
Identification of StSnRK2 members in potato
A genome-wide search using SnRK2 cDNA sequences of Arabidopsis, maize and rice as references to identify the eight SnRK2 homologous sequences in potato genome (Solanum tuberosum group Phureja DM1–3). The gene-specific primers were designed to clone the eight SnRK2s homologous cDNA from the potato cultivar ‘Longshu 3’. The candidate sequences coding potato SnRK2s ranged from 1008 bp to 1089 bp (Table 1). The eight sequences were designated as StSnRK2.1, StSnRK2.2 to StSnRK2.8 and the confirmed full length cDNA were submitted to NCBI. The length of the predicted coding sequences of potato SnRK2s ranged from 37.7 4 kDa to 41.5 kDa. The protein length varied from 335 (StSnRK2.1) to 362 amino acids (StSnRK2.3). The protein length and the molecular weight of StSnRK2s were similar to SnRK2 members reported in Arabidopsis, rice and maize (Table 1, Additional file 2: Figure S1) [14].
Table 1.
The basic properties and characteristics of StSnRK2 genes in potato
| Gene | Chra | NCBI GIb | Genomic locusc | Positiond | Cds (bp)e | Exons (No)f | Full lengh (bp)g | Amino acid (aa)h | Theoretical pIi | Mw (kDa)j |
|---|---|---|---|---|---|---|---|---|---|---|
| StSnRK2.1 | 4 | 404,435,142 | PGSC0003DMT400079214 | 56,031,065…56,034,492 | 1008 | 9 | 3428 | 335 | 5.37 | 37.74 |
| StSnRK2.2 | 8 | 404,435,144 | PGSC0003DMT400067424 | 37,772,908…37,775,887 | 1020 | 9 | 4028 | 339 | 5.93 | 38.33 |
| StSnRK2.3 | 1 | 404,435,146 | PGSC0003DMT400066617 | 77,595,506...77599207 | 1089 | 9 | 3702 | 362 | 4.96 | 41.06 |
| StSnRK2.4 | 1 | 404,435,148 | PGSC0003DMT400061170 | 73,495,432...73500028 | 1083 | 9 | 4597 | 360 | 5.52 | 41.49 |
| StSnRK2.5 | 4 | 404,435,150 | PGSC0003DMT400060762 | 6,332,105...6336032 | 1035 | 9 | 3928 | 344 | 5.76 | 39.20 |
| StSnRK2.6 | 5 | 404,435,152 | PGSC0003DMT400060263 | 46,960,739...46964637 | 1077 | 7 | 3899 | 358 | 6.08 | 41.20 |
| StSnRK2.7 | 12 | 404,435,154 | PGSC0003DMT400045810 | 53,346,801...53350990 | 1011 | 9 | 4190 | 336 | 5.57 | 38.40 |
| StSnRK2.8 | 11 | 404,435,156 | PGSC0003DMT400041671 | 4,225,503…4,229,063 | 1050 | 9 | 2561 | 349 | 4.88 | 39.85 |
aChromosome in which target gene is located in potato. bGenebank index number in NCBI. cGenomic locus in potato Genome Sequencing Consortium database (http://potatogenome.net/index.php/Main_Page). dThe position of genes on the corresponding list in ‘Genomic locus’. eLength of coding region in base pairs. fThe numbers of exons which were analyzed with splign (http://www.ncbi.nlm.nih.gov/sutils/splign). gNucleotide accession of full-length cDNA in potato Genome Sequencing Consortium database. hAmino acid number of deduced protein. iThe Theoretical pI of deduced protein. jThe molecular weight of deduced protein.
Based on the potato genome sequence, StSnRK2 genes were located on different chromosomes (Table 1) with StSnRK2.1 and StSnRK2.5 on chromosome 4, StSnRK2.3 and StSnRK2.4 on chromosome 1, and StSnRK2.2, StSnRK2.6, StSnRK2.7 and StSnRK2.8 on chromosomes 8, 5, 12 and 11, respectively.
Gene and protein structures of StSnRK2s
All StSnRK2s had nine exons except for StSnRK2.6 with seven exons (Fig. 1), and the first exon was around 120 bp except for StSnRK2.3 with a 177 bp first exon. The seven genes with nine exons had highly conserved exon lengths, with the length of the second through the eighth exons 75 bp, 102 bp, 54 bp, 93 bp, 93 bp, 105 bp and 99 bp, respectively. StSnRK2.6 had seven exons, and the length of the second exon showed a possible combination of the second to fourth conserved exons of the other StSnRK2 genes. The predicted secondary structure of proteins suggested that eight StSnRK proteins might share a high degree of similarity (Table 2).
Fig. 1.
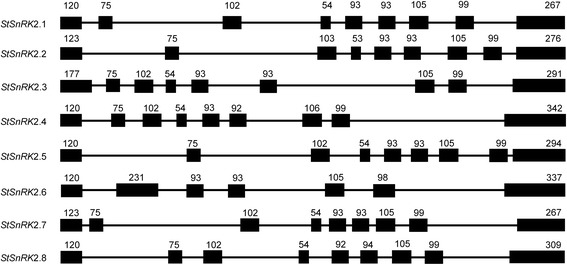
Gene structures of StSnRK2s in potato. Introns and exons were represented by lines and filled boxes, respectively. The numbers above the exons indicate the length (bp) of the exons
Table 2.
The secondary structure of StSnRK2 protein sequences. The secondary structure of the deduced polypeptide was predicted using the programs of SOPMA which listed in Expasy (www.EXPASY.org)
| Gene | Alpha helix (%) | Extended strand (%) | Beta turn (%) | Random coil (%) |
|---|---|---|---|---|
| StSnRK2.1 | 42.39 | 16.42 | 7.46 | 33.73 |
| StSnRK2.2 | 43.36 | 16.22 | 6.78 | 33.63 |
| StSnRK2.3 | 38.12 | 17.40 | 7.46 | 37.02 |
| StSnRK2.4 | 47.22 | 15.56 | 9.17 | 28.06 |
| StSnRK2.5 | 34.59 | 19.19 | 6.69 | 39.53 |
| StSnRK2.6 | 43.02 | 13.41 | 5.31 | 38.27 |
| StSnRK2.7 | 43.45 | 15.48 | 4.17 | 36.90 |
| StSnRK2.8 | 38.68 | 16.62 | 7.16 | 37.54 |
Phylogenetic analysis of StSnRK2
The N-termini of StSnRK2s were almost identical, but the C-terminal regions were highly divergent (ranging from 261 to 327 aa) (Fig. 2). All StSnRK2s had a conserved Ser/Thr protein kinase domain in the N-terminal (4 to 260 aa). Multiple alignment analysis based on the full length amino acid sequences of 37 SnRK2s was conducted to understand the evolutionary relationship between StSnRK2s and other reported SnRK2s. An unrooted phylogenetic tree was constructed with the amino acid sequences, using the neighbor-joining method (Fig. 3 and Additional file 2: Figure S2). The results demonstrated that SnRK2s are highly conserved in the plant kingdom. StSnRK2s were related to each other and could be divided into three distinct groups. The StSnRK2.1, StSnRK2.2, StSnRK2.5, StSnRK2.7 and StSnRK2.8 belonged to group I, StSnRK2.4 and StSnRK2.6 to group II and StSnRK2.3 alone to group III.
Fig. 2.
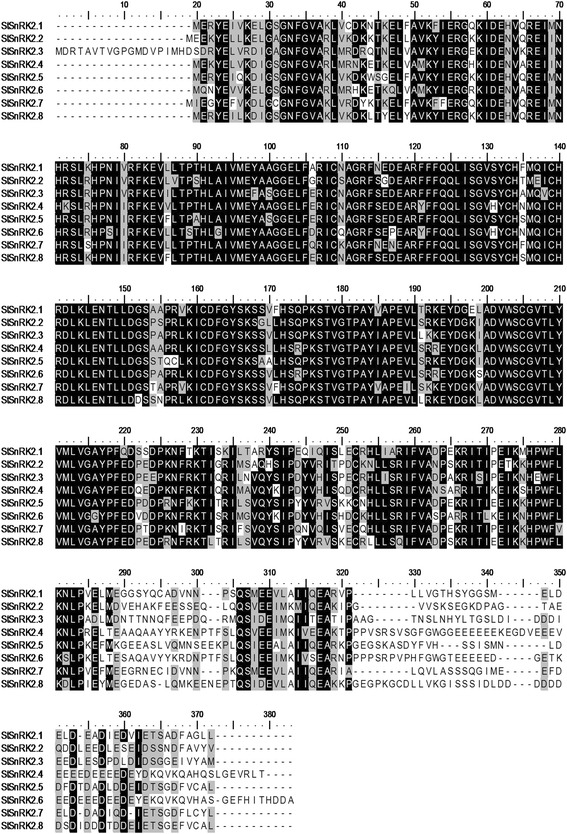
Alignment of the amino acid sequences of StSnRK2s. Identical amino acid residues are covered by black, similar residues are indicated by gray and the gaps in the sequences are indicated by dashes
Fig. 3.
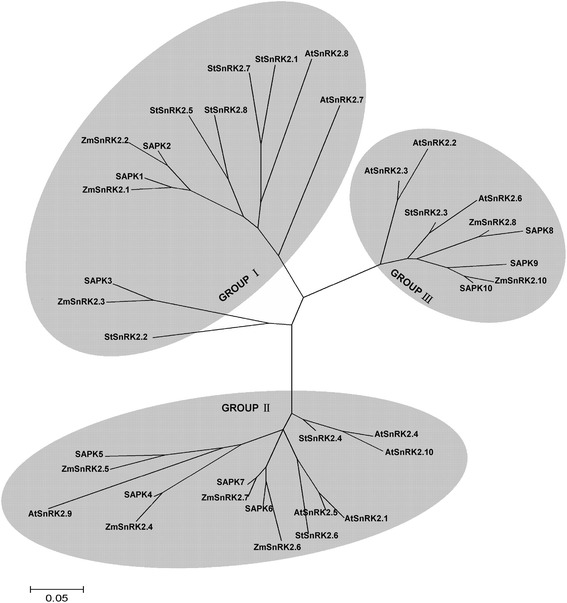
A phylogenetic tree constructed with CluxtalX1.8 using the SnRK2s full length amino acid sequence from potato, Arabidopsis, rice and maize. The bootstrap analysis was performed using 1000 replicates in MEGA (5.0) to evaluate the reliability of different phylogenetic groups
Subcellular localization of StSnRK2 proteins
To better understand the functions of StSnRK2, we transiently expressed pBEGFP-StSnRK2 fusion proteins in onion epidermal cells (Fig. 4). The epidermal cells were transformed with the 35S: GFP observed with fluorescence microscopy. Transiently expressed GFP-StSnRK2 fusion proteins were all detected in the nucleus or cytoplasm of onion epidermal cells. The fluorescence of GFP fused with StSnRK2.1, StSnRK2.2, StSnRK2.6, StSnRK2.7, and StSnRK2.8 was detected in the nucleus and cytoplasm of onion epidermal cells. On the other hand, StSnRK2.3 and StSnRK2.4 are mainly associated to the nucleus, while StSnRK2.5 seems to be associated to subcellular organelles.
Fig. 4.
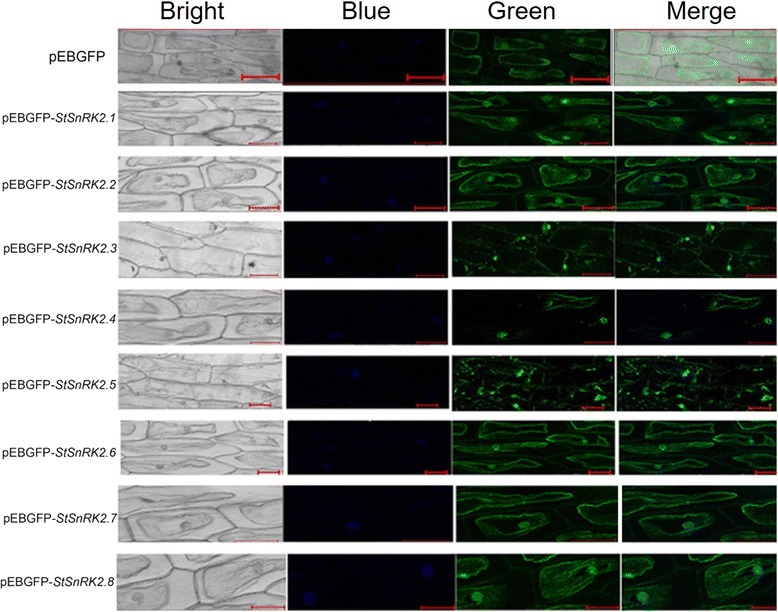
pBEGFP-StSnRK2 protein targeted to nucleus and cytoplasm in onion epidermal cells. The pEBGFP and pEBGFP-StSnRK2s plasmids were introduced into Agrobacterium tumefaciens strain EHA105 and were used for transformation into onion epidermal cells. Onion epidermal tissues were subsequently incubated on MS solid nutrient medium in the dark at 26 °C for 48 h. Localization of fluorescent proteins in onion epidermal cells were observed by a Zeiss LSM 710/ConfoCor2 laser-scanning imaging system (CarlZeiss, Jena, Germany). Fluorescence was detected between 505 and 550 nm with excitation at 488 nm. GFP fluorescence and light field vision were recorded in separate channels and then merged into an overlay image. Bar = 50 μm
Tissue-specific expression of StSnRK2 genes
The relative expressions of the eight StSnRK2 genes varied among tissues (root, leaf, stem and tuber) (Fig. 5). StSnRK2.1, StSnRK2.2, StSnRK2.5 and StSnRK2.6 were highest expressed in root. In contrast, the expression levels of StSnRK2.3, StSnRK 2.7 and StSnRK 2.8 in leaf and stem were significantly higher than those in root (P < 0.05). StSnRK2.4 expression in leaf, stem and tuber were significantly higher than that in root. The expressions of StSnRK2.1, StSnRK2.2, StSnRK2.5, StSnRK2.6, StSnRK2.7 and StSnRK2.8 were lower in tubers than those in root.
Fig. 5.
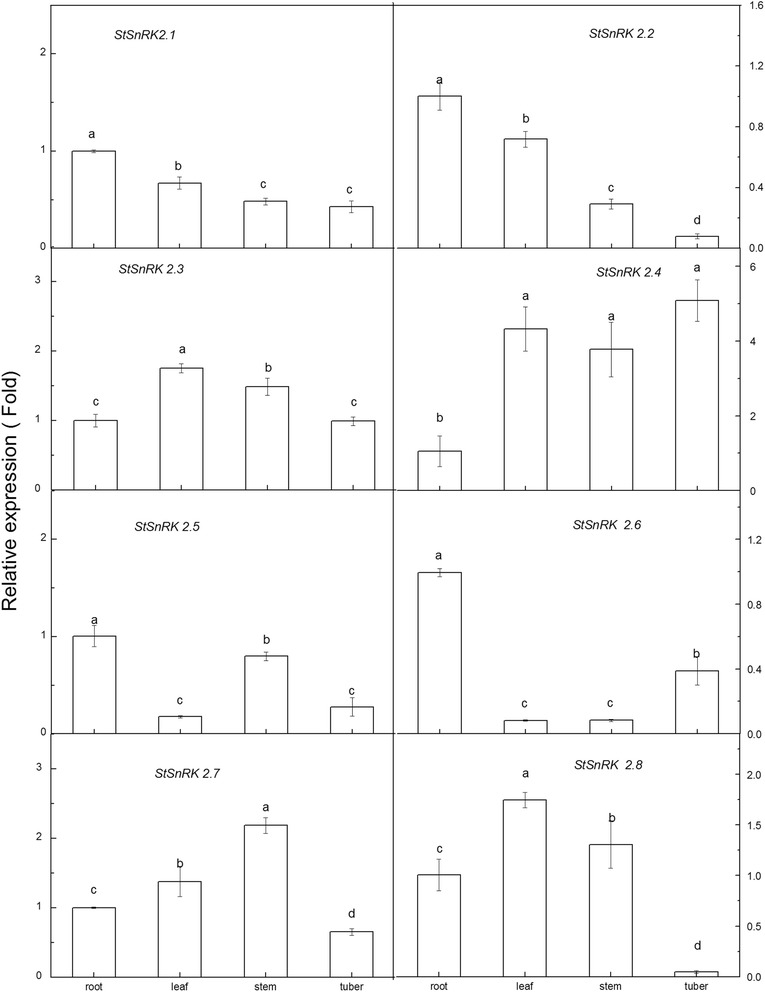
Results are presented as differential relative transcript abundance of StSnRK2.1 to StSnRK2.8 in different tissues; data represent the means ± SD of three replicates and different letters indicate significant difference at P < 0.05. Total RNA was extracted from 60-day-old plants. Y-axis showed the transcript fold to that in roots
Stress-induced expression of StSnRK2s and physiological responses of potato plants
Potato plantlets were treated with H2O (Control), ABA (50 μM), NaCl (200 mM) and PEG-6000 (5%). The total soluble sugar and proline contents were analyzed after 0 h, 2 h, 4 h, 6 h, 12 h, 24 h and 48 h. The results indicated that ABA (50 μM), NaCl (200 mM) and PEG-6000 (5%) treatments increased the total soluble sugar and proline with prolonging of treatments (Additional file 2: Figure S3). The relative gene expression of StSnRKs was analyzed using qRT-PCR. The results showed that the expression of all genes rose firstly after NaCl treatment, but different genes showed different responses to the stress condition (Fig. 6). StSnRK2.1 and StSnRK2.7 were similar and had their relative expression levels were highest at 12 h after subjected to the stress. Their expression levels gradually declined after 12 h, but StSnRK2.1 had less reduction, and was significantly higher at 48 h, while StSnRK2.7 declined greatest. The StSnRK2.2, StSnRK2.4 and StSnRK2.6 had similar expression trends. The relative expression levels of StSnRK2.5 and StSnRK2.8 increased rapidly after 2 h of treatments, and then began to fall to the levels lower than the control after the 6 h, and remained at a relatively low expression. The relative expression level of StSnRK2.3 increased significantly 4 h after the stress, then began to decrease, but remained significantly high from 6 to 24 h and fell to the control level after 48 h of treatments, possibly indicating they have various signaling pathways.
Fig. 6.
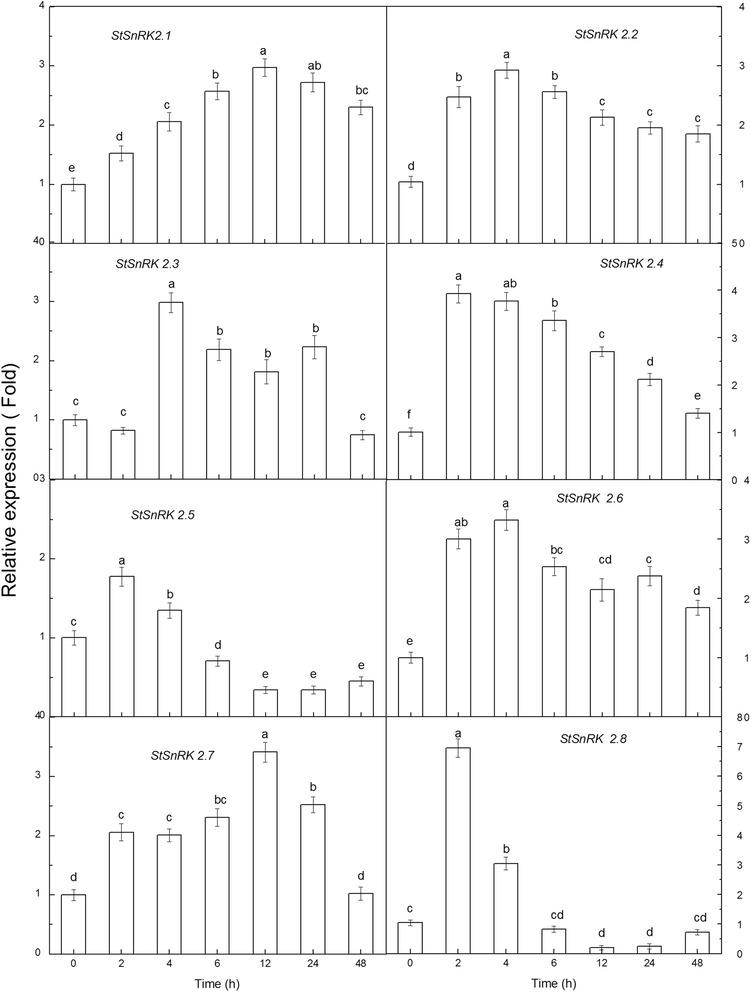
The differential relative transcript expression level of StSnRK2.1 to StSnRK2.8 under 200 mM NaCl stress treatments; data represent the means ± SD of three replicates and different letters indicate significant difference at P < 0.05. Total RNA was extracted from four-week-old plants subjected to 200 mM NaCl stress treatments for 2–48 h. Y-axis showed the transcript fold to that in the CK
Under PEG (5%) treatment, the relative expression of StSnRK2.1, StSnRK2.2 and StSnRK2.3 rapidly rose after 2 h–4 h of treatment, and remained a relative stable level until 48 h (Fig. 7). StSnRK2.1 relative expression increased significantly after 4 h, but the relative expression levels of StSnRK2.2 and StSnRK2.3 increased rapidly after 2 h of treatment. The relative expression levels of StSnRK2.4 and StSnRK2.5 rose rapidly, about five times of the control and reached a peak at 2 h of treatment, then declined significantly. The relative expression of StSnRK2.6 declined slowly with the prolonging of treatment time; however, the relative expression of StSnRK2.8 was about seven times of the control at 4 h of treatment and then declined after 6 h of exposure to stress. StSnRK2.7 expression slowly increased with time until 12 h of treatment, then decreased significantly with no obvious difference compared to control at 24 h of treatment and to less than half of the control at 48 h of treatment.
Fig. 7.
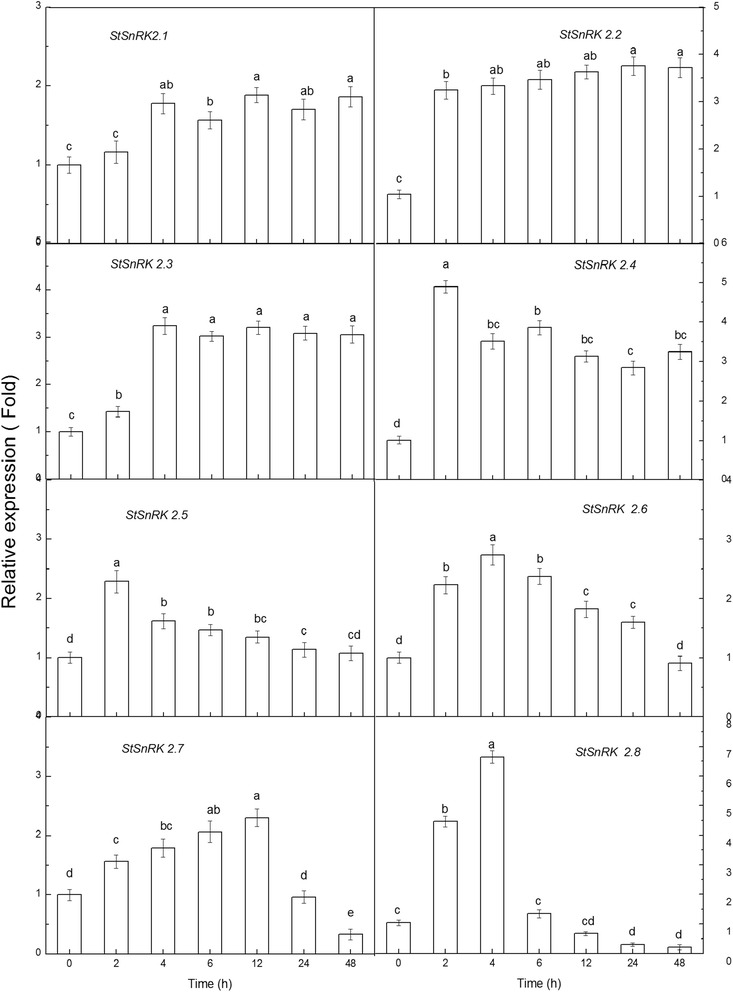
The differential relative transcript expression level of StSnRK2.1 to StSnRK2.8 under 5% PEG stress treatments; data represent the means ± SD of three replicates and different letters indicate significant difference at P < 0.05. Total RNA was extracted from four-week-old plants subjected to 5% PEG stress treatments for 2–48 h. Y-axis showed the transcript fold to that in the CK
The relative expression pattern of StSnRK2 genes under ABA treatment were different from NaCl and PEG treatments (Fig. 8). After 2 h of ABA, the relative expression level of StSnRK2.3 declined slightly, increased to a maximum after 6 h and then remained stable. Other members had no obvious changes in expression under ABA treatment, although the expression levels of StSnRK2.1, StSnRK2.5 and StSnRK2.7 declined slightly at 2 h, but gradually returned to the control levels after 4 h of treatment. Compared to NaCl and PEG treatments, there was no significantly increasing trend. After 2 h of treatment, the expression levels of StSnRK2.2 and StSnRK2.4 also increased, but those of StSnRK2.6 and StSnRK2.8 had not changed. The results showed that StSnRK2 genes have no obvious response to ABA; most members of the subfamily may be involved in plant response pathways to stresses and were ABA-independent except that StSnRK2.3 is ABA-dependent.
Fig. 8.
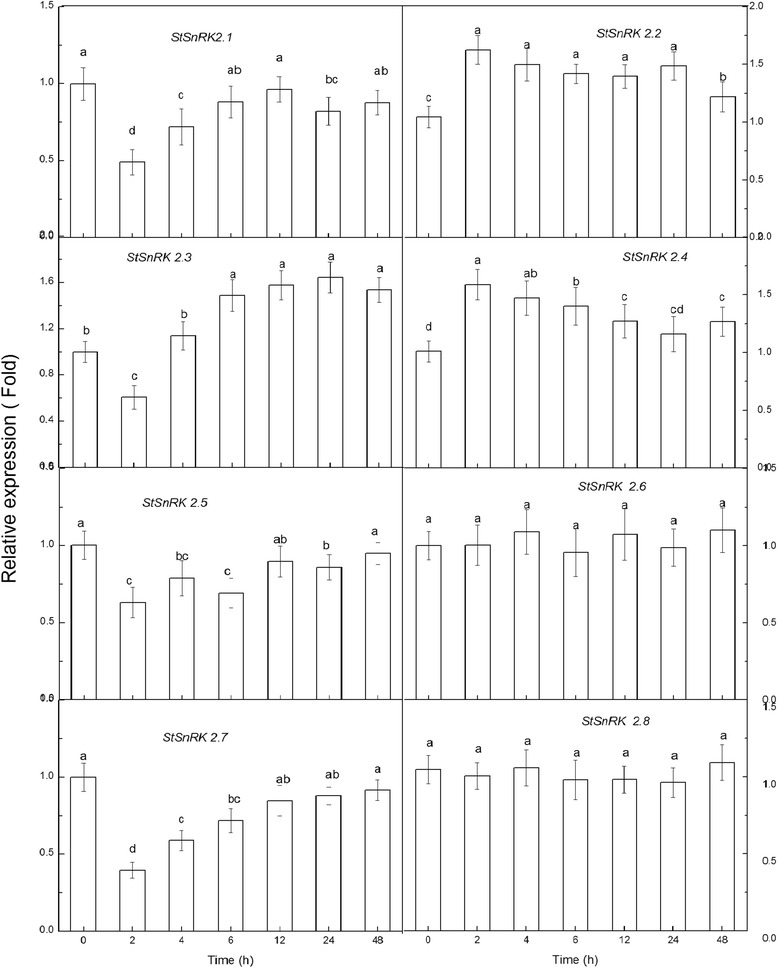
The differential relative transcript expression level of StSnRK2.1 to StSnRK2.8 under 50 μM ABA stress treatments, the data represent the means ± SD of three replicates and different letters indicate significant difference at P < 0.05. Total RNA was extracted from four--week-old plants subjected to 50 μM ABA stress treatments for 2–48 h. Y-axis showed the transcript fold to that in the CK
To identify the presence of cis-elements in the promoter regions of the StSnRK2s, the 2 kb length of the upstream region was considered as the promoter region and used to search for stress-responsive elements. We cloned eight StSnRK2 genes from the potato cultivar ‘Longshu-3’ and analyzed their location in the genome, gene structure, amino acid sequence divergence and tissue specific and stress-induced expression patterns. All genes except for StSnRK2.4 carried ABRE, StSnRK2.1, StSnRK2.2 and StSnRK2.4 carried DRE/CRT and StSnRK2.1, StSnRK2.2, StSnRK2.4, StSnRK2.5 and StSnRK2.7 had LTRE.
Discussions
The SnRK2 subfamily is an osmotic-stress activated protein kinase and has been identified and characterized in Arabidopsis, rice, maize and other plant species. Many studies have showed that SnRK2 members have potential roles in improving stress tolerance and increase crop yields [29, 32]. In the present study, we identified and cloned eight StSnRK2 genes from potato cultivar ‘Longshu-3’, and analyzed their genome distribution, gene structure, phylogenic and tissue specific and stress-induced expression patterns. Similar to SnRK2s in Arabidopsis, rice and maize, StSnRK2 genes in potato were distributed over several chromosomes. All the StSnRK2s except for StSnRK2.6 have nine exons, similar to the SnRK2s in other plant species [26, 46]. The functional characteristics of the C-terminal region of SnRK2s has been reported in several plant species like Arabidopsis [4, 5, 13, 14, 16, 17, 35], rice [25, 36] and maize [26, 47]. The evolutionary distance of StSnRK2.3 is very close to AtSnRK2.6 (Fig. 3). The gene products of AtSnRK2.2 and AtSnRK2.3 are mostly concentrated in leaves, contributing largely to the ABA-dependent stomatal regulation [4, 15].
Tissue-specific expression of SnRK2 genes were observed in many species. For example, fava bean AAPKs induced by ABA signaling were mainly expressed in guard cells [48]. The expression of SAPK members is different among tissues in rice: SAPK8, 9 and 10 are regulated only by ABA in blades and roots, with the level of expression in roots higher than in other tissues [25]. Root tips, as a dynamic and specialized tissue, play a crucial role in sensing water and nutrients and often have rapid responses by transmitting appropriate signals. In current study, the expressions of StSnRK2s in various tissues of potato plantlets were highly diversified and these proteins may mediate various metabolic processes under normal growing conditions. The expression of StSnRK2.4 was higher in shoot tissues than in roots, and could be induced by NaCl, ABA and PEG (Figs. 5, 6, 7, 8), suggesting that StSnRK2.4 may be sensitive to stress signals and function in osmotic-stress responses in shoot tissues of potato. The expression of TaSnRK2.4 was stimulated by high salt treatments, and the overexpression of TaSnRK2.4 in Arabidopsis enhanced salt tolerance [28]. StSnRK2.4 and AtSnRK2.4 were classified into the same group, implying that StSnRK2.4 may be a potential target for improving the salt tolerance of potato. In Arabidopsis, SnRK2.2, SnRK2.3 and SnRK2.6 are typically activated by ABA, and can phosphorylate the ABA-responsive elements. So, they are important for the activation of ABA-responsive genes. In rice, SAPK8, 9, and 10 are activated by ABA [25]. The regulation of the plant responses to ABA via SnRK2s pathways occurs by direct phosphorylation of various downstream targets, for example, SLAC1, KAT1, AtRbohF and transcription factors were required for the expression of numerous stress response genes [46]. The expression of ZmSnRK2 genes can be induced by various stress treatments, suggesting their potential roles in stress responses. We found that each gene of StSnRK2 subfamily has a unique expression pattern in different tissues of potato, with the expressions of StSnRK2.2 and StSnRK2.8 very low in roots, whereas the expressions of StSnRK2.5 and StSnRK2.6 were very low in leaves. However, they were insensitive to ABA treatments, probably due to the different regulatory elements that each StSnRK2 carried on the 2-kb upstream region (Table 3). Alternatively, the concentration of ABA and the prolonging of the ABA treatment need to be further discussed. The manipulation of StSnRK2s can be a valuable approach for improving the stress tolerance of potato.
Table 3.
Putative Cis elements existed in the 2 kb upstream region of StSnRK2 genes
| Name | ABRE | DRE/CRT | LTRE |
|---|---|---|---|
| StSnRK2.1 | 4 | 1 | 1 |
| StSnRK2.2 | 3 | 2 | 1 |
| StSnRK2.3 | 1 | 0 | 0 |
| StSnRK2.4 | 0 | 2 | 2 |
| StSnRK2.5 | 2 | 0 | 2 |
| StSnRK2.6 | 2 | 0 | 0 |
| StSnRK2.7 | 3 | 0 | 1 |
| StSnRK2.8 | 5 | 0 | 0 |
The sequence of ABRE elements include ACGTG, MACGYGB, TACGTGTC, YACGTGGC, and CCACGTGG. The sequence of DRE/CRT element include RCCGAC, ACCGAC, ACCGAGA and GTCGAC. The sequences of LTRE element include CCGAC, CCGAAA, ACCGACA and CCGAC
Conclusions
In the present study, we identified and characterized eight SnRK2 genes in the potato genome. The eight StSnRK2s exhibit similar gene structure and secondary structures in potato to the SnRK2s found in other plant species. The fluorescence of GFP fused with StSnRK2.1, StSnRK2.2, StSnRK2.6, StSnRK2.7, and StSnRK2.8 was detected in the nucleus and cytoplasm of onion epidermal cells. On the other hand, StSnRK2.3 and StSnRK2.4 are mainly associated to the nucleus, while StSnRK2.5 seems to be associated to subcellular organelles. The relative expression of the eight SnRK2 genes varied among tissues (roots, leaves, tubers and stems) and abiotic stresses (ABA, NaCl and PEG-6000) with the prolonging of treatments. This study provides valuable information for the future functional dissection of potato SnRK2 genes in stress signal transduction, plant growth and development studies.
Additional files
Table S1 and Table S2. Table S1. The Accession numbers of SnRK2s from Arabidopsis, rice and maize. Table S2. Oligonucleotides used for StSnRK2 cloning and qRT-PCR. (DOCX 21 kb)
Figure S1, Figure S2 and Figure S3. Figure S1. Alignment of the amino acid sequences of the SnRK2s from Arabidopsis, rice, maize and potato. Identical amino acids residues are covered by black, similar residues are indicated by gray, Dashes indicate gaps in the sequences to allow maximal alignment. Figure S2. The polygenetic tree was constructed with (CluxtalX1.8) using the SnRK2s full length amino acid sequence from potato. The bootstrap values are in percentage. Figure S3. The proline and total soluble sugar content under 200 mM NaCl, 5% PEG, and 50 μM ABA treatments. Data represent the means ± SD of three replicates and different letters indicate significant difference at P < 0.05. (DOCX 1796 kb).
Acknowledgments
We would like to thank Professor Yantai Gan for valuable discussion on the conception of the manuscript.
Funding
This research was supported by the Key Project of Gansu Province Programs for Fundamental Research and Development (grant No. 1102NKDA025), the National Natural Science Foundation of China (grant No. 31460369) and Longyuan Young Scientist Supporting Program.
Availability of data and materials
All data and supporting information from this paper will be available and freely distributed by corresponding authors.
Authors’ contributions
JB was in charge of experiment design and manuscript prepareation; JM was responsable for the gene cloning, qRT-PCR experiments and data analysis; HY was responsable for subcellular localization of StSnRK2 proteins; AK contributed in the data analysis and manuscript preparation; AF, SL and HG helped with the sample preparation and data collection and analysis; Junlian Zhang and DW contributed to the lab orgnization and supervision of the overall progress of the project. Jinlin Zhang contributed to the experimental design, data analysis and manuscript writing and revision. All the authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
No human participants, human data or human tissue were involved in this study. No animals were used in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ABA
Abscisic acid
- NaCl
Sodium chloride
- PEG-6000
Polyethyleneglycol-6000
- qRT-PCR
Quantitative real-time PCR
- SnRK
Sucrose non-ferment 1 related protein kinase
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12863-017-0506-6) contains supplementary material, which is available to authorized users.
Contributor Information
Jiangping Bai, Phone: +86 931-7632021, Email: baijp@gsau.edu.cn.
Jinlin Zhang, Phone: +86 931-8912357, Email: jlzhang@lzu.edu.cn.
References
- 1.Hu XJ, Zhang ZB, Xu P, Fu ZY, Hu SB, Song WY. Multifunctional genes: the cross-talk among the regulation networks of abiotic stress responses. Biol Plant. 2010;54:213–223. doi: 10.1007/s10535-010-0039-6. [DOI] [Google Scholar]
- 2.Thapa G, Dey M, Sahoo L, Panda SK. An insight into the drought stress induced alterations in plants. Biol Plant. 2011;55:603–613. doi: 10.1007/s10535-011-0158-8. [DOI] [Google Scholar]
- 3.Laurie S, Halford NG. The role of protein kinases in the regulation of plant growth and development. Plant Growth Regul. 2001;34:253–265. doi: 10.1023/A:1013311807626. [DOI] [Google Scholar]
- 4.Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, Shinozaki K, Hirayama T. ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol. 2006;140:115–126. doi: 10.1104/pp.105.070128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii H, Verslues PE, Zhu JK. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell. 2007;19:485–494. doi: 10.1105/tpc.106.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Movahed S, Sayed Tabatabaei BE, Alizade H, Ghobadi C, Yamchi A, Khaksar G. Constitutive expression of Arabidopsis DREB1B in transgenic potato enhances drought and freezing tolerance. Biol Plant. 2012;56:37–42. doi: 10.1007/s10535-012-0013-6. [DOI] [Google Scholar]
- 7.Hong L, Hu B, Liu X, He CY, Yao Y, Li XL, Li L. Molecular cloning and expression analysis of a new stress-related AREB gene from Arachis hypogaea. Biol Plant. 2013;57:56–62. doi: 10.1007/s10535-012-0236-6. [DOI] [Google Scholar]
- 8.Qin F, Shinozaki K, Yamaguchi-Shinozaki K. Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant Cell Physiol. 2011;52:1569–1582. doi: 10.1093/pcp/pcr106. [DOI] [PubMed] [Google Scholar]
- 9.Bing L, Feng CC, Li JL, Li XX, Zhao BC, Shen YZ, Huang ZJ, Ge R. Overexpression of the AtSTK gene increases salt, PEG and ABA tolerance in Arabidopsis. J Plant Biol. 2013;56:375–382. doi: 10.1007/s12374-013-0154-y. [DOI] [Google Scholar]
- 10.Tao XC, Lu YT. Loss of AtCRK1 gene function in Arabidopsis thaliana decreases tolerance to salt. J Plant Biol. 2013;56:306–314. doi: 10.1007/s12374-012-0352-z. [DOI] [Google Scholar]
- 11.Halford NG, Hardie DG. SNF1-related protein kinases: glob al regulators of carbon metabolism in plants? Plant Mol Biol. 1998;37:735–748. doi: 10.1023/A:1006024231305. [DOI] [PubMed] [Google Scholar]
- 12.Ghillebert R, Swinnen E, Wen J, Vandesteene L, Ramon M, Norga K, Rolland F, Winderickx J. The AMPK ⁄ SNF1 ⁄ SnRK1 fuel gauge and energy regulator: structure, function and regulation. FEBS J. 2011;278:3978–3990. doi: 10.1111/j.1742-4658.2011.08315.x. [DOI] [PubMed] [Google Scholar]
- 13.Boudsocq M, Barbier-Brygoo H, Lauriere C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyper osmotic and saline stresses in Arabidopsis thaliana. J Biol Chem. 2004;279:41758–41766. doi: 10.1074/jbc.M405259200. [DOI] [PubMed] [Google Scholar]
- 14.Umezawa T, Yoshida R, Maruyama K, Yamaguchi-Shinozaki K, Shinozaki K. SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2004;101:17306–17311. doi: 10.1073/pnas.0407758101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii H, Zhu JK. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA. 2009;106:8380–8385. doi: 10.1073/pnas.0903144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii H, Verslues PE, Zhu JK. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc Natl Acad Sci USA. 2011;108:1717–1722. doi: 10.1073/pnas.1018367108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun L, Zhang M, Ren J, Qi JX, Zhang GJ, Leng P. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 2010;10:257–267. doi: 10.1186/1471-2229-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B, Wang L, Sopko MS, Yao C, Yau K, Burton S, Zhuang M, McCaskill DG, Gachotte D, Thompson M, Greene TW. The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol. 2010;153:99–113. doi: 10.1104/pp.109.150789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RR, Wagner RL, Verhey SD, Walker-Simmons MK. The abscisic acid-responsive kinase PKABA1 interacts with a seed-specific abscisic acid response element-binding factor, TaABF, and phosphorylates TaABF peptide sequences. Plant Physiol. 2002;130:837–846. doi: 10.1104/pp.001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakashima K, Fujita Y, Kanamori N, Katagiri T, Umezawa T, Kidokoro S, Maruyama K, Yoshida T, Ishiyama K, Kobayashi M, Shinozaki K, Yamaguchi-Shinozaki K. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 2009;50:1345–1363. doi: 10.1093/pcp/pcp083. [DOI] [PubMed] [Google Scholar]
- 22.Anderberg RJ, Walker-Simmons MK. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc Natl Acad Sci USA. 1992;89:10183–10187. doi: 10.1073/pnas.89.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Assmann SM. An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell. 1996;8:2359–2368. doi: 10.1105/tpc.8.12.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLoughlin F, Galvan-Ampudia CS, Julkowska MM, Caarls L, Does D, Lauriere C, Munnik T, Haring MA, Testerink C. The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J. 2012;72:436–449. doi: 10.1111/j.1365-313X.2012.05089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattor T. Differential activation of the rice sucrose nonfermenting1–related protein kinase 2 family by hyperosmotic stress and abscisic acid. Plant Cell. 2004;16:1163–1177. doi: 10.1105/tpc.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huai JL, Wang M, He JG, Zheng J, Dong ZG, Lv HK, Zhao JF, Wang GY. Cloning and characterization of the SnRK2 gene family from Zea mays. Plant Cell Rep. 2008;28:1861–1868. doi: 10.1007/s00299-008-0608-8. [DOI] [PubMed] [Google Scholar]
- 27.Kelne A, Pekala I, Kaczanowski S, Muszynska G, Hardie DG, Dobrowolska G. Biochemical characterization of the tobacco 42-kD protein kinase activated by osmotic stress. Plant Physiol. 2004;136:3255–3265. doi: 10.1104/pp.104.046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao XG, Zhang HY, Tian SJ, Chang XP, Jing RL. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J Exp Bot. 2010;61:683–696. doi: 10.1093/jxb/erp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang HY, Mao XG, Jing RL, Chang XP, Xie HM. Characterization of a common wheat (Triticum aestivum L.) TaSnRK2.7 gene involved in abiotic stress responses. J Exp Bot. 2011;62:975–988. doi: 10.1093/jxb/erq328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li LB, Zhang YR, Liu KC, Ni ZF, Fang ZJ, Sun QX, Gao JW. Identification and bioinformatics analysis of SnRK2 and CIPK family genes in Sorghum. Agr Sci China. 2010;9:19–30. doi: 10.1016/S1671-2927(09)60063-8. [DOI] [Google Scholar]
- 31.Monks DE, Aghoram K, Courtney PD, DeWald DB, Dewey RE. Hyperosmotic stress induces the rapid phosphorylation of a soybean phosphatidylinositol transfer protein homolog through activation of the protein kinases SPK1 and SPK2. Plant Cell. 2001;13:1205–1219. doi: 10.1105/tpc.13.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Ji W, Gao P, Li Y, Cai H, Bai X, Chen Q, Zhu Y. GsAPK, an ABA-activated and calcium-independent SnRK2-type kinase from G. soja, mediates the regulation of plant tolerance to salinity and ABA stress. PLoS One. 2012;7:e33838. doi: 10.1371/journal.pone.0033838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamauchi D, Zentella R, Ho TD. Molecular analysis of the barley (Hordeum vulgare L.) gene encoding the protein kinase PKABA1 capable of suppressing gibberellin action in aleurone layers. Planta. 2002;215:319–326. doi: 10.1007/s00425-002-0740-6. [DOI] [PubMed] [Google Scholar]
- 34.Boneh U, Biton I, Schwartz A, Ben-Ari G. Characterization of the ABA signal transduction pathway in Vitis vinifera. Plant Sci. 2012;187:89–96. doi: 10.1016/j.plantsci.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Mizoguchi M, Umezawa T, Nakashima K, Kidokoro S, Takasaki H, Fujita Y, Yamaguchi-Shinozakj K, Shinozaki K. Two closely related subclass II SnRK2 protein kinases cooperatively regulate drought-inducible gene expression. Plant Cell Physiol. 2010;51:842–847. doi: 10.1093/pcp/pcq041. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi Y, Murata M, Minami H, Yamamoto S, Kagaya Y, Hobo T, Yamamoto A, Hattori T. Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 2005;44:939–949. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 37.Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plantarum. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 38.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapustin Y, Souvorov A, Tatusova T, Lipman D. Splign: algorithms for computing spliced alignments with identification of paralogs. Biol Direct. 2008;3:1–13. doi: 10.1186/1745-6150-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Willems E, Leyns L, Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal Biochem. 2008;379:127–129. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 44.Zhang SJ, Li N, Gao F, Yang AF, Zhang JR. Over-expression of TsCBF1 gene confers improved drought tolerance in transgenic maize. Mol Breed. 2010;26:455–465. doi: 10.1007/s11032-009-9385-5. [DOI] [Google Scholar]
- 45.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 46.Kulik A, Wawer I, Krzywińska E, Bucholc M, Dobrowolska G. SnRK2 protein kinases—key regulators of plant response to abiotic stresses. OMICS. 2011;15:859–872. doi: 10.1089/omi.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ying S, Zhang DF, Li HY, Liu YH, Shi YS, Song YC, Wang TY, Li Y. Cloning and characterization of a maize SnRK2 protein kinase gene confers enhanced salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2011;30:1683–1699. doi: 10.1007/s00299-011-1077-z. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Wang XQ, Watson MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 and Table S2. Table S1. The Accession numbers of SnRK2s from Arabidopsis, rice and maize. Table S2. Oligonucleotides used for StSnRK2 cloning and qRT-PCR. (DOCX 21 kb)
Figure S1, Figure S2 and Figure S3. Figure S1. Alignment of the amino acid sequences of the SnRK2s from Arabidopsis, rice, maize and potato. Identical amino acids residues are covered by black, similar residues are indicated by gray, Dashes indicate gaps in the sequences to allow maximal alignment. Figure S2. The polygenetic tree was constructed with (CluxtalX1.8) using the SnRK2s full length amino acid sequence from potato. The bootstrap values are in percentage. Figure S3. The proline and total soluble sugar content under 200 mM NaCl, 5% PEG, and 50 μM ABA treatments. Data represent the means ± SD of three replicates and different letters indicate significant difference at P < 0.05. (DOCX 1796 kb).
Data Availability Statement
All data and supporting information from this paper will be available and freely distributed by corresponding authors.


