Fig. 8.
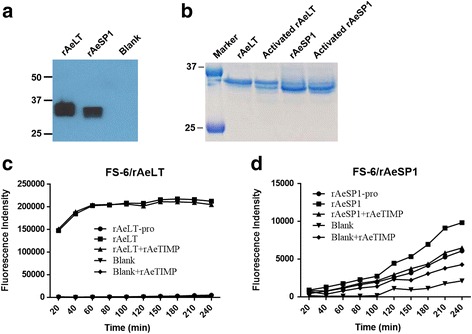
Drosophila S2 cell expression of two putative serine collagenase genes and catalytic activities of the recombinant proteins. a Western blot detection of recombinant (r)AeLT and (r)AeSP1 proteins using an His tag-specific monoclonal antibody. Blank, S2 cells were transfected with non-insert containing plasmid vector; rAeLT and rAeSP1, S2 cells were transfected with plasmid vectors expressing AeLT or AeSP1. Molecular masses in kDa are indicated. b SDS-PAGE showing purified rAeLT and rAeSP1 with or without additional trypsin activation (pro-protein cleavage). Molecular masses in kDa are indicated. Kinetics of rAeLT (c) and rAeSP1 (d) in vitro activities and their inhibition by rAeTIMP. Twenty ng of purified rAeLT or rAeSP1 were preincubated with/without TPCK-treated trypsin for 15 min at RT, then incubated with 20 ng of rAeTIMP, or reaction buffer (RB) at RT for 2 h, followed by addition of FS-6 substrate. Fluorescence intensity was measured every 20 min. rAeLT-pro and rAeSP1-pro, pro-protein of rAeLT and rAeSP1; rAeLT and rAeSP1, activated rAeLT and rAeSP1
