Fig. 7.
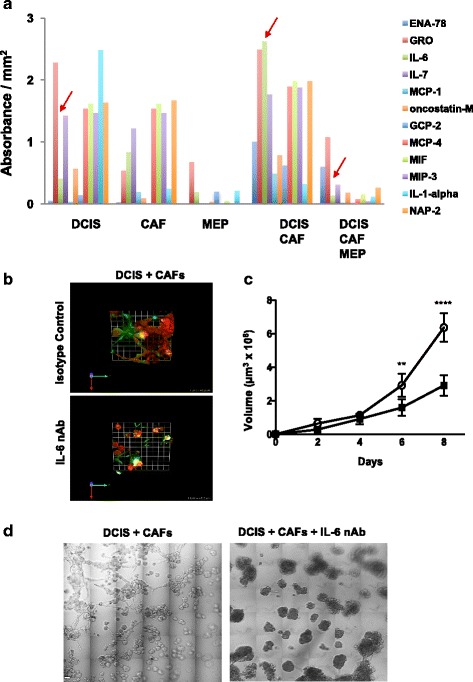
Targeting interleukin 6 (IL-6) reduces size and invasiveness of and extracellular matrix (ECM) degradation by ductal carcinoma in situ/cancer-associated fibroblast (DCIS-CAF) structures formed in mammary architecture and microenvironment engineering (MAME) cocultures. a Secretion of cytokines was assessed in 8-day conditioned media with a RayBio G5 human cytokine antibody array (RayBiotech, Norcross, GA, USA). Secretion of IL-6 was significantly elevated (p = 0.002 as determined by one-way analysis of variance [ANOVA]) when MCF10.DCIS (DCIS) cells were cocultured with WS-12T (CAFs), but it was not reduced when cocultured with myoepithelial cell (MEPs) or with CAFs plus MEPs (arrows). b MCF10.DCIS-lenti-RFP (DCIS) and WS-12T (CAFs) cells were seeded onto reconstituted basement membrane (rBM) overlaid with 2% rBM in the presence of isotype control or 100 ng/ml IL-6 neutralizing antibody (nAb) and imaged live at day 8. Representative en face views of 3D reconstructions of DCIS (red)-CAF (unlabeled) structures and associated degraded dye-quenched collagen IV (dDQ-IV; green) in MAME cultures; areas of colocalization appear yellow-white. One grid unit = 45 μm. c Volume of structures formed in MAME cultures of DCIS and CAFs in the presence of isotype control (open circles) or 100 ng/ml IL-6 nAb (filled squares) at days 2, 4, 6, and 8. Volume of structures was measured with Volocity software (n = 4). Data represent mean ± SD. ** p ≤ 0.01 and **** p ≤ 0.0001 as determined by two-way ANOVA. d Differential interference contrast images of MCF10.DCIS (DCIS) and WS-12T (CAFs) MAME cultures in the presence of isotype control or 100 ng/ml IL-6 nAb at day 8. Images are 36 contiguous tiled fields. Scale bar = 80 μm. Images are representative of at least three independent experiments. Additional results are shown in Additional file 14: Figure S7. ENA-78 Epithelial-derived neutrophil-activating peptide 78, GRO Growth-related oncogene, MCP Monocyte chemoattractant protein, GCP-2 Granulocyte chemotactic protein 2, MIF Macrophage migration inhibitory factor, MIP-3 Macrophage inflammatory protein 3, NAP-2 Neutrophil-activating protein 2
