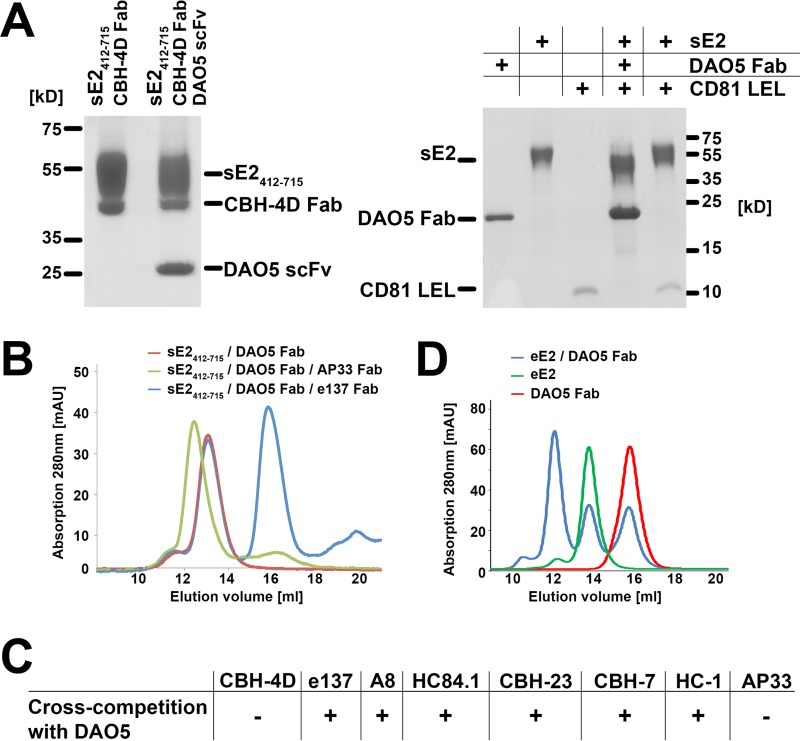
FIG 3 .
Cross-competition profile of DAO5. (A) Cross-competition and biochemical analysis of the sE2412-715–DAO5 complex. (Left) sE2412-715 and an sE2412-715–DAO5 scFv complex were affinity loaded onto a StrepTactin column, a CBH-4D Fab fragment was added, and the eluted complex was analyzed by SDS-PAGE under nonreducing conditions. (Right) CD81-LEL was incubated overnight at RT with the full-length HCV ectodomain (comprising residues 384 to 715; sE2) and the sE2-DAO5 Fab complex, followed by SEC and analysis of the peak fractions by SDS-PAGE under reducing conditions. DAO5 heavy and light chains form an apparent single band in the reducing gel due to an almost identical molecular mass of ~24 kDa. (B) A preformed sE2412-715–DAO5 Fab complex was incubated in the absence or presence of a Fab fragment targeting a non-overlapping (AP33) or overlapping (e137) region of cE2 and analyzed by SEC. After preincubation with AP33 (green), appearance of a peak at a higher molecular mass indicated ternary complex formation; after preincubation with e137 Fab (blue), the presence of peaks corresponding to the binary complex (at ~13 ml) and an isolated Fab fragment (at ~16 ml) showed that no ternary complex was formed. (C) Cross-competition profile of the sE2412-715–DAO5 Fab complex, obtained by SEC analysis as described for panel B, with a panel of Fab fragments derived from the indicated well-characterized anti-E2 MAbs. (D) sE2384-713 produced in human cells (eE2) was incubated with DAO5 Fab, and then the complex as well as the individual components were analyzed by SEC. The presence of a peak at a higher molecular mass indicated binary complex formation (12 ml, blue). A considerable protein fraction eluted in peaks corresponding to uncomplexed eE2 (green) and DAO5 Fab (red).