FIG 1 .
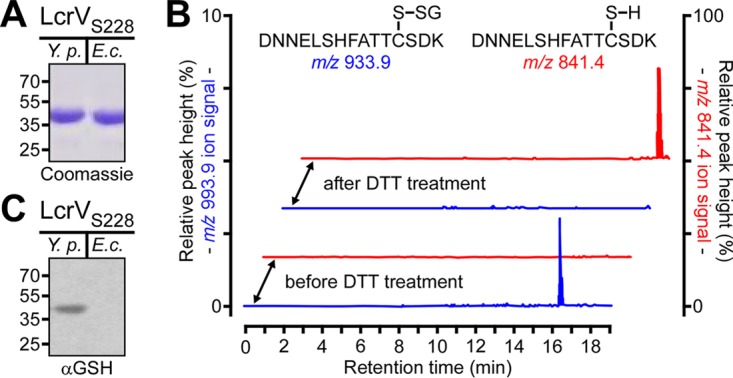
LcrV secreted by Y. pestis is glutathionylated at Cys273. (A) Coomassie-stained SDS-PAGE of LcrVS228 purified via Strep-Tactin affinity chromatography from either the culture supernatant of Y. pestis KLD29(pKG48) (Y.p.) or cell lysates of E. coli DH5α(pKG48) (E.c.). (B) Reconstructed ion traces for the unmodified (m/z 841.4) [(M+2H)2+ ion of 1,680.71] and glutathione-modified (m/z 993.9) [(M+2H)2+ ion of 1,985.79] tryptic peptides from the Y. pestis LcrVS228 protein encompassing Cys273 before and after treatment with dithiothreitol (DTT). The chromatograms show the absence of the unmodified peptides and the presence of the glutathione-modified peptides before treatment and following DTT treatment, the presence of the unmodified peptides, and the absence of the glutathione-modified peptides. (C) LcrVS228 purified from Y. pestis culture supernatants or E. coli extracts (rLcrVS228) was analyzed by immunoblotting with glutathione-specific antiserum (αGSH).
