Table 2.
Substrate scope for the syntheses of compounds 4 and 6.a
| Entry | Starting materials | GBB product 4 | Yieldb (%) | Cyclized product 6 | Yieldb (%) |
| 1 | 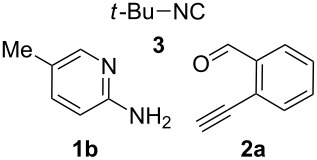 |
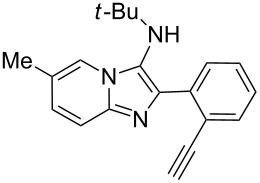 4b |
94 |
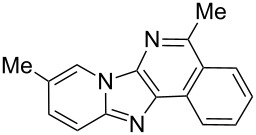 6b |
72 |
| 2 | 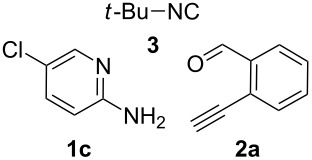 |
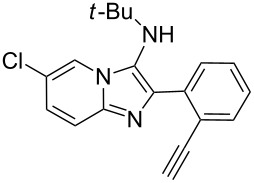 4c |
80 |
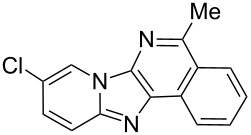 6c |
75 |
| 3 | 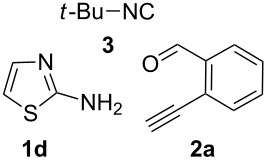 |
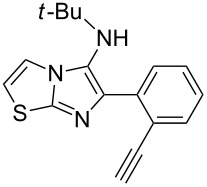 4d |
62 |
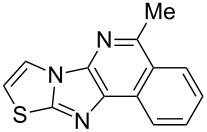 6d |
61 |
| 4 | 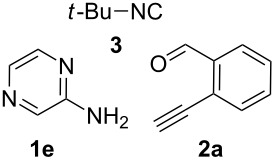 |
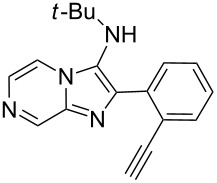 4e |
96 |
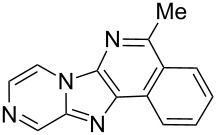 6e |
63 |
| 5 | 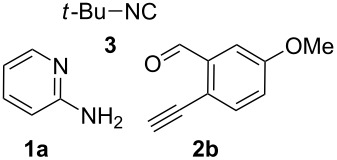 |
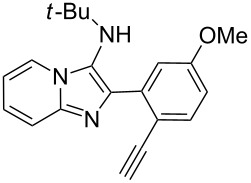 4f |
89 |
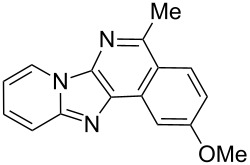 6f |
78 |
| 6 | 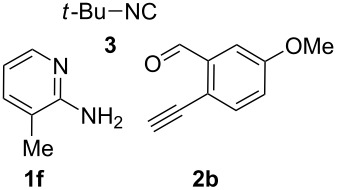 |
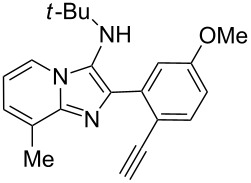 4g |
61 |
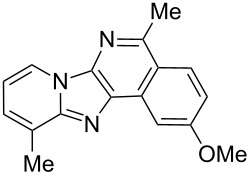 6g |
80 |
| 7 | 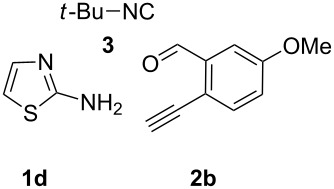 |
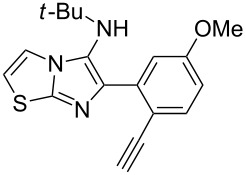 4h |
85 |
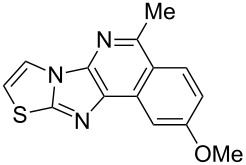 6h |
56 |
| 8 | 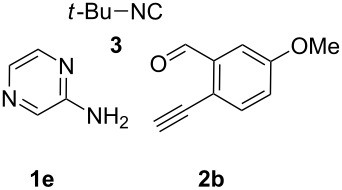 |
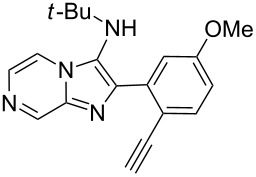 4i |
88 |
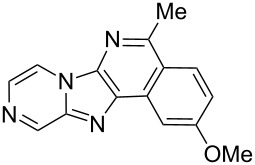 6i |
58 |
| 9 | 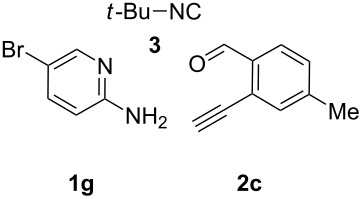 |
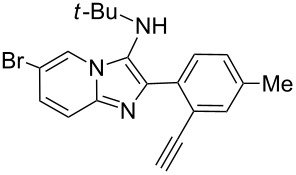 4j |
64 |
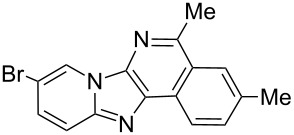 6j |
78 |
| 10 | 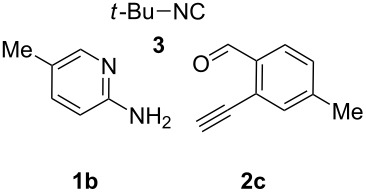 |
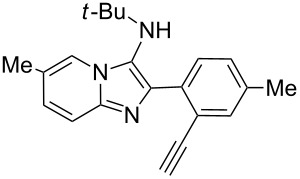 4k |
75 |
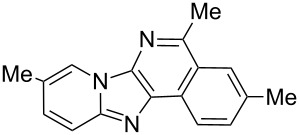 6k |
87 |
| 11 | 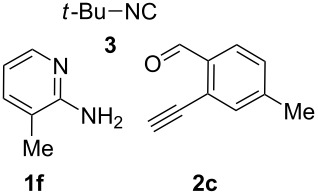 |
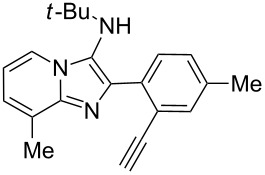 4l |
49 |
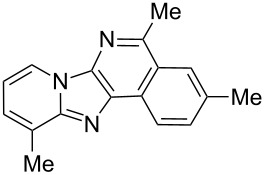 6l |
79 |
| 12 | 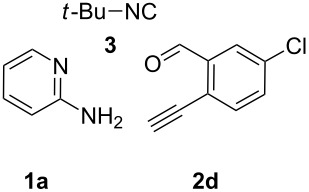 |
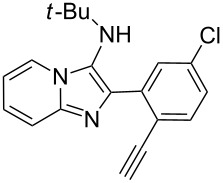 4m |
71 |
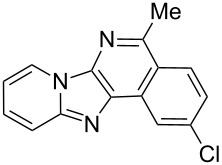 6m |
48 |
| 13 | 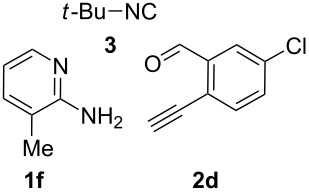 |
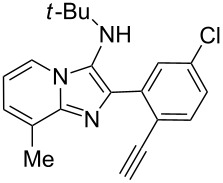 4n |
47 |
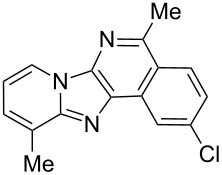 6n |
55 |
| 14 | 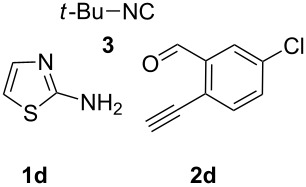 |
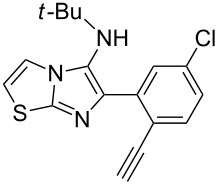 4o |
54 |
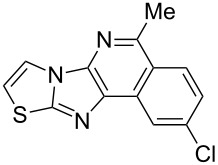 6o |
62 |
| 15 | 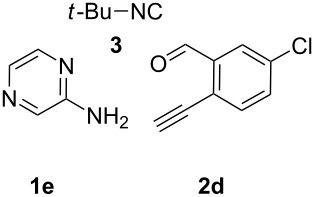 |
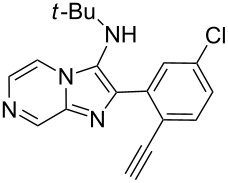 4p |
95 |
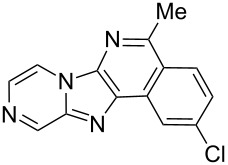 6p |
63 |
| 16 | 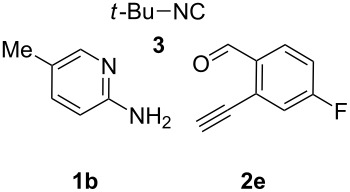 |
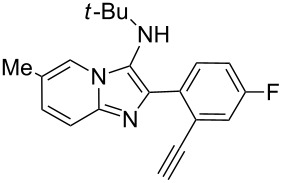 4q |
74 |
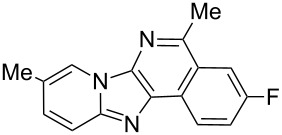 6q |
58 |
| 17 | 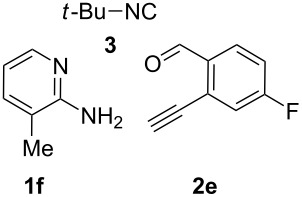 |
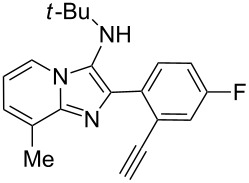 4r |
43 |
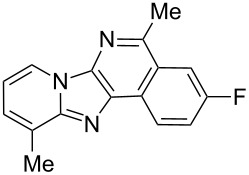 6r |
67 |
| 18 | 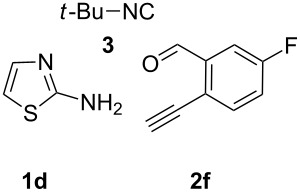 |
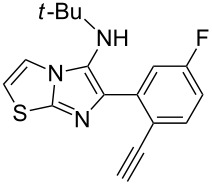 4s |
57 |
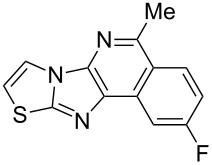 6s |
59 |
aGBB reaction conditions: 1 (0.5 mmol), 2 (0.5 mmol), 3 (0.6 mmol), MeOH (1 mL); PTSA (5%), room temperature, 12h; annulation conditions: substrate 4 (0.2 mmol), Au(JohnPhos)Cl (10 mol %), CH3CN (2 mL) at reflux temperature for 24 h. bIsolated yields.
