Abstract
Aim: To evaluate the effectiveness of different irrigation solutions and ultrasonic activation of the irrigation solutions on the removal of calcium hydroxide (Ca(OH)2) from the simulated immature root canals after apexification.
Materials and methods: One-hundred and one single-rooted teeth were used. The root canals were shaped with ProTaper rotary files up to F5. Simulation of roots with immature apices was carried out using size 4 Unicore drills. An injectable Ca(OH)2 was injected into each root canal, and packed to the working length. Then, cotton pellets were placed over canal orifices, and apical and coronal parts of the roots were sealed with resin-modified glass ionomer cement, and light cured. Specimens were stored in distilled water for 3 months at 37°C. After 3 months, the temporary coronal seal was removed and the samples were randomly divided into: (a) saline (n = 20), (b) ultrasonic activation of saline (n = 20), (c) sodium hypochlorite (NaOCl) (n = 20), (d) ultrasonic activation of NaOCl (n = 15), (e) chlorhexidine digluconate (CHX) (n = 20) and one positive control group (n = 3) and one negative control group (n = 3). The amount of remaining Ca(OH)2 on the canal walls was measured under stereomicroscope with 30× magnification. Comparisons between groups were made by the non-parametric Kruskal-Wallis test and Dunn post-test at a significance level of p < 0.05.
Results: There were no significant differences among the saline, ultrasonic activation of saline, NaOCl, ultrasonic activation of NaOCl and CHX (p > 0.05) groups.
Conclusions: Irrigation solutions and ultrasonic activation of the irrigation solutions could not completely remove Ca(OH)2 from the simulated immature root canals.
Keywords: Irrigation solutions, immature teeth, NaOCl, saline, CHX, ultrasonic irrigation
Introduction
Calcium hydroxide (Ca(OH)2) has been used as a root canal medicament due to its antimicrobial activity and organic tissue dissolution capacity.[1,2] Another use of Ca(OH)2 is in apexification treatment due to its ability to initiate hard tissue formation.[1] Its success rate reaches up to 100%, especially in apexification treatment.[3–5] Ca(OH)2 dissociates into calcium and hydroxyl ions, and the calcium ions have a remineralization capacity, [6] while the hydroxyl ions have an antimicrobial action capacity.[7] However, Ca(OH)2 has certain disadvantages affecting the success of root canal treatment. The remaining Ca(OH)2 in dentin tubules influences the bond strength of dentin,[8–10] might react with the root canal sealer chemically reducing the flow and working time of the sealer,[11] effects the penetration of the root canal sealers into the dentin tubules[12] and the quality of the sealing of root canal filling,[13] and increases the micro-leakage of root canal fillings.[13] Also, Ca(OH)2 interacts with zinc oxide eugenol-based sealers which causes the sealer to set inconsistently.[14] Ideally, the complete removal of residual Ca(OH)2 from the root canals and dentin tubules is a primary goal before final obturation of the canal.
Lambrianidis et al.[15,16] described instrumentation with a master apical file (MAF) followed by irrigation of the root canals as the most effective method for the removal of Ca(OH)2 from the root canal. Salgado et al.[17] described that combined usage of MAF and irrigant removed Ca(OH)2 better than irrigant alone. Lee et al.[18] described passive ultrasonic irrigation (PUI) as the most effective method for the removal of Ca(OH)2 from the root canal. Balvedi et al.[19] compared the syringe injection and PUI for the removal of Ca(OH)2 from the root canals and found no difference between them. Nandini et al.[20] described 17% ethylenediaminetetraacetic acid (EDTA) and 10% citric acid as the most effective materials for the removal of the powder form of Ca(OH)2. Kenee et al. reported that rotary instrument and ultrasonic techniques removed significantly more Ca(OH)2 than irrigant alone.[21] van der Sluis et al. described[22] that passive ultrasonic irrigation with 2% sodium hypochlorite (NaOCl) removed significantly more Ca(OH)2 from artificial root canal grooves than syringe delivery of 2% NaOCl or water as an irrigant.
A wide range of techniques and solutions have been used to date for the removal of Ca(OH)2 from the root canals of mature teeth,[15–22] but no in vitro study has been found in the literature for the removal of Ca(OH)2 from the root canals after apexification treatment of immature teeth. The aim of the present study is to evaluate and compare the removal of Ca(OH)2 from the root canals of immature teeth after 3 months using NaOCl, saline, ultrasonic activation of NaOCl, ultrasonic activation of saline and chlorhexidine digluconate (CHX).
Materials and methods
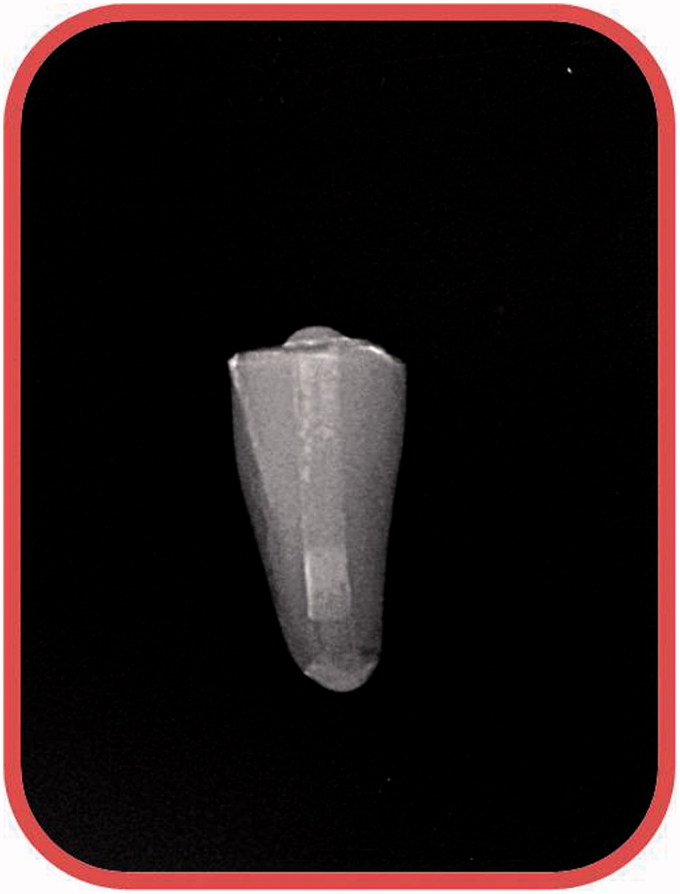
This study was approved by the Institutional Review Board of Sifa University, Izmir, Turkey (Reference number 180-54). On- hundred and one freshly extracted human single-rooted teeth with straight roots were used in this study. The teeth were stored in 5% NaOCl for 2 days at 37 °C. The calculus and soft tissue debris of the teeth were removed with ultrasonics and immersed in 10% formalin solution until use. All samples were examined under a stereomicroscope at 10× magnification to ensure that no caries, fractures, cracks, root deformities, resorption and calcification existed on the root walls. Teeth with such findings were excluded from the study, and replaced by similar teeth and a completely formed apex. Then, the crowns were removed with a water-cooled diamond bur to obtain approximately 13 mm length from the apex. The roots were measured with a digital caliper (Mitutoyo, Hampshire, UK) to standardize their length. The working length was determined with 15 K file by subtracting 1 mm beyond the apex. The root canals were shaped with Protaper rotary files up to F5 file size. Simulation of roots with immature apices were carried out using size 4 green 1.5 mm diameter Unicore drills (Ultradent Products, Inc., South Jordan, UT). During preparation, the canals were irrigated with 5% NaOCl using a 27-gauge slot-tripped needle after each file. After instrumentation, a final canal rinse was made using 5 ml of 17% EDTA for 1 min and 5 ml of 5% NaOCl for 1 min followed by the final rinse with 5 ml distilled water. Finally, the root canals were dried using paper points. Then, Ca(OH)2 (Calcicure calcium hydroxide paste, Cuxhaven, Germany, Lot 1329431, 2015-06) was injected into each root canal and packed to the working length with a lentulo spiral. Radiographs were taken in order to confirm complete filling of the canals (Figure 1). Then, cotton pellets were placed over canal orifices, and apical and coronal parts of the roots were sealed with resin-modified glass ionomer cement (Ionoseal, Cuxhaven, Germany, Lot 1333165, 2015–08), and light cured. These specimens were stored in 100% relative humidity for 3 months at 37 °C. After 3 months, temporary coronal seal was removed and the samples were randomly divided into five experimental groups according to the method used for Ca(OH)2 removal: one positive control group (n = 3) and one negative control group (n = 3). These groups are as follows.
Figure 1.
Radiographs were taken in order to confirm complete filling of the canals.
Positive control
Ca(OH)2 was not removed from the root canals (n = 3).
Negative control
Root canals were not filled with Ca(OH)2 (n = 3).
Group saline
Irrigation needles were placed at two-third of the working length and roots were irrigated for 3 min with 15 ml saline solution using a syringe with 27-gauge needle. Finally, the root canals were dried using paper points.
Group ultrasonic saline
The root canals were ultrasonically irrigated for 3 min with 15 ml of saline solution. Ultrasonic irrigation was performed using the ultrasonic device (EMS, Le Sentier, Switzerland). A smooth ultrasonic file (size 15: 0.02 taper) (ESI instrument, EMS, Le Sentier, Switzerland) was placed within 1 mm of the working length and irrigation began almost at the same time. Finally, the root canals were dried using paper points.
Group NaOCl
Irrigation needles were placed at two-third of the working length and root canals were irrigated for 3 min with 15 ml of 5% NaOCl solution using a syringe with a 27-gauge needle. Finally, the root canals were dried using paper points.
Group ultrasonic NaOCl
The root canals were ultrasonically irrigated for 3 min with 15 ml of 5% NaOCl solution. Ultrasonic irrigation was performed using an ultrasonic device (EMS, Le Sentier, Switzerland). A smooth ultrasonic file (size 15: 0.02 taper) (ESI instrument, EMS, Le Sentier, Switzerland) was placed within 1 mm of the working length, and irrigation began almost at the same time. Finally, the root canals were dried using paper points.
Group CHX
Irrigation needles were placed at two-third of the working length and root canals were irrigated for 3 min with 15 ml 2% CHX solution using a syringe with a 27-gauge needle. Finally, the root canals were dried using paper points.
The buccal and lingual surfaces of roots were cut with a diamond saw under dry conditions, thus preserving the inner layer of dentin around the canal, and then roots were split using a lancet and a hammer so that the root halves were obtained.
The amount of remaining Ca(OH)2 on the canal walls was measured under a stereomicroscope ((Nikon type102, Tokyo, Japan) with 30× magnification by one examiner, scored in mm2 and recorded as a percentage of the overall canal surface area. The scoring system was as follows: score 0, clean root canal walls; score 1, 0–10% of the root canal walls covered by Ca(OH)2; score 2, 11–20% of the root canal walls covered by Ca(OH)2; score 3, 21–50% of the root canal walls covered by Ca(OH)2; score 4, 51–99% of the root canal walls covered by Ca(OH)2; score 5, root canal walls completely covered by Ca(OH)2.
Statistical analysis
All the data were analyzed using the SPSS software package (Statistical Package for Social Sciences, version 11.5, SPSS Inc., Chicago, IL). Comparisons between groups were made by the non-parametric Kruskal–Wallis test and Dunn post-test at a significance level of p < 0.05.
Results
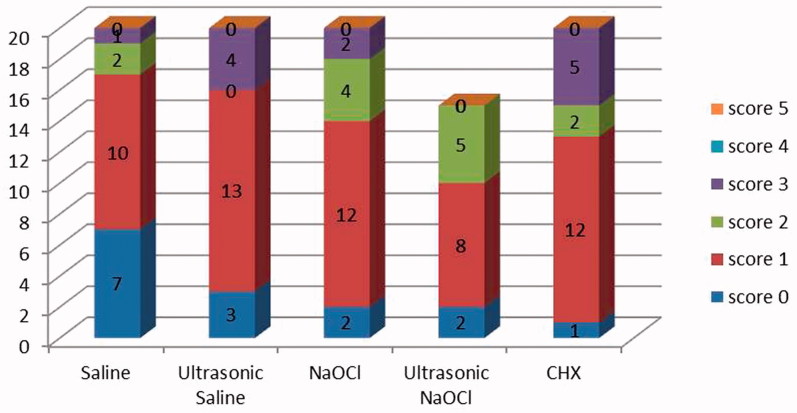
The negative controls had no residues on the dentinal walls and the positive controls had the root canals completely filled with Ca(OH)2. There were no significant differences between groups saline, ultrasonic saline, NaOCl, ultrasonic NaOCl and CHX (p > 0.05). Figure 2 shows the distribution of the scores for the removal of Ca(OH)2 from the root canals. Scores 4 and 5 were not found, and score 1 was more frequent in all experimental groups. Image representing the experimental groups is shown in Figure 3.
Figure 2.
The distribution of the scores for the removal of Ca(OH)2 from the root canals.
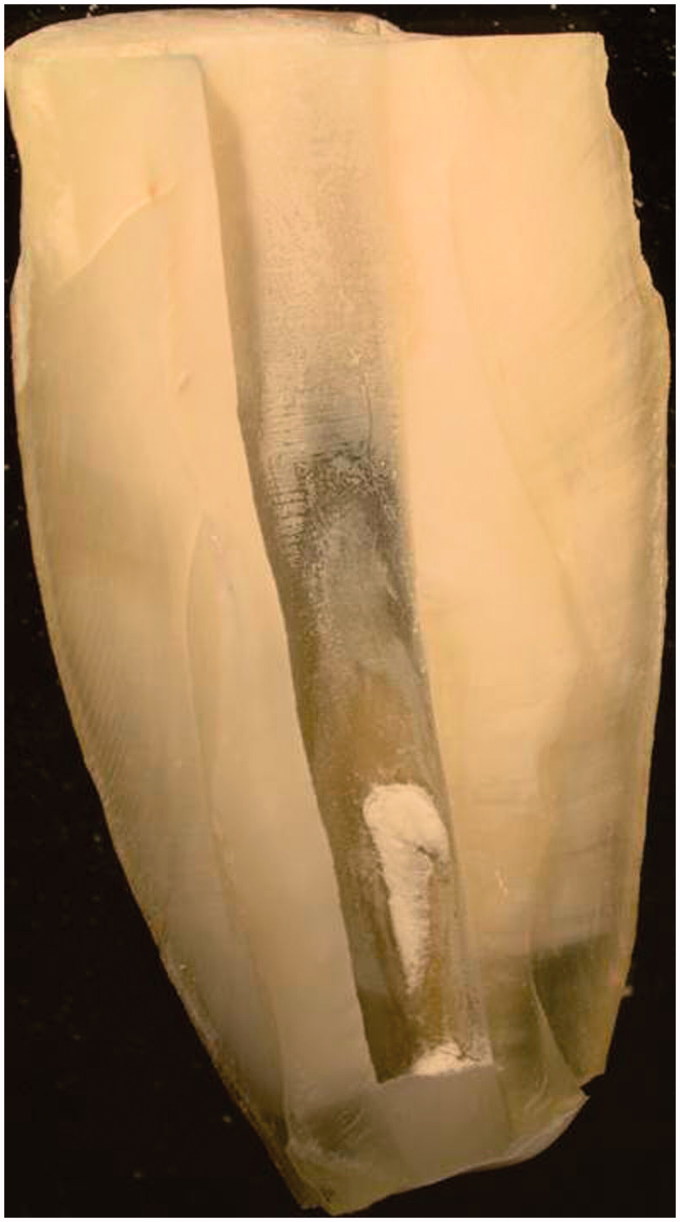
Figure 3.
The Ca(OH)2 residues persistence on the root canal wall dentin (Score 1).
Discussion
The remnants of Ca(OH)2 on the canal walls could affect the success of the root canal treatment, and they should be removed from the root canals before final obturation.[11] In the present study, we compared the ability of several irrigation solutions on the removal of Ca(OH)2 from the simulated immature root canal walls after 3 months placement.
We used simulated immature teeth in our present study, and the advantage of this model is the standardization of the width and length of the root canal space. The disadvantage of this model is that the standardized canal size might not accurately reflect the natural root canal system with respect to complex anatomy, grooves, isthmuses and canal irregularities. Thus, it may be easier to remove Ca(OH)2 from simulated immature teeth than from natural immature teeth.
We evaluated the removal of Ca(OH)2 from the root canals with different techniques. In some studies the amount of residual Ca(OH)2 in the root canal was evaluated by measuring the surface area of the residue on the canal walls in terms of mm2,[19,21,23] however in other studies, a scoring method has been used.[22,24] In the present study we used two techniques: first, surface area of the residual Ca(OH)2 in the canals was measured in terms of percentile values, and second, these values were scored from zero to five.
Nandini et al.[20] stated that 17% EDTA was highly effective for removing Ca(OH)2 from the root canals. We did not use EDTA for the removal of Ca(OH)2 from the root canals because clinically, EDTA might damage the hard tissue formed after apexification.[25]
A groove model was not made in the present study because standardized grooves may not reflect the entire anatomy of root canals.[26] To standardize the irrigation solution, 15 ml solution was used for 3 min for each tooth.
In the present study, residual Ca(OH)2 on the canal walls was determined under the stereomicroscope at 30× magnification, hence only the superficial layer of Ca(OH)2 could be measured, however, Ca(OH)2 may have remained inside the dentinal tubules. Therefore, the evaluation of the remaining Ca(OH)2 should be performed with a three-dimensional view or advanced microscopic techniques.
According to the results of this study, irrigation solutions and ultrasonic activation of the NaOCl and saline could not completely remove the residual Ca(OH)2 from the root canals of immature teeth. These results were similar to the findings of the previous studies performed with mature root canals.[15,16,21,22]
CHX has an antimicrobial activity against Enterococcus Faecalis, which has been isolated from failed endodontic retreatment cases and persistent infections of the root canals. [27] In the present study, CHX did not completely remove the residual Ca(OH)2 from the root canals.
Taşdemir et al.[23] stated that ultrasonic agitation of NaOCl removed more Ca(OH)2 than NaOCl alone. van der Sluis et al.[22] indicated that ultrasonic irrigation with 2% NaOCl was more effective than syringe irrigation of 2% NaOCl. Baldevi et al.[19] stated that syringe irrigation of saline and ultrasonic activation of saline solution could not completely remove Ca(OH)2 from the root canals. In the present study we used immature teeth, and ultrasonic activation of saline and NaOCl could not completely remove Ca(OH)2 from the root canals. As statistically significant difference was not found between syringe irrigation and ultrasonic activation of saline and NaOCl on the removal of Ca(OH)2 from the root canals, we might suggest that there is no need for using ultrasonic devices for the removal of Ca(OH)2 from the immature teeth after apexification treatment.
Rödig et al.[26] stated that smaller apical preparation size might affect hydrodynamics and decrease the effectiveness of root canal irrigation solutions. Also, several studies[28–30] have reported that larger apical preparation size increased the mechanical efficacy of root canal irrigation solutions. In the present study, our samples were immature teeth and apical preparation size was large, but none of the solutions could completely remove Ca(OH)2 from the root canals.
Recently, agitation of the root canal irrigation solution with a laser technique called a photon-induced photoacoustic streaming (PIPS) is being used for the removal of the dentin debris from the root canals.[31,32] Arslan et al.[33] stated that PIPS was more effective than ultrasonic irrigation for the removal of the dentinal debris from the root canals. The results of our study would have been different if we used PIPS, and its effectiveness could be studied in the future.
We found that neither irrigation solutions nor ultrasonic activation of the irrigation solutions could completely remove the residual Ca(OH)2 from the root canals of immature teeth. Capar et al.[34] stated that the use of the Self-Adjusting File (SAF) system with root canal irrigation solution might increase the removal of Ca(OH)2 from the root canals. Kuga et al.[35] evaluated the effectiveness of two types of rotary instruments on the removal of Ca(OH)2 from the root canals and found that ProTaper F1 instrument was more effective than K3 Endo rotary instrument. We might suggest that the combination of rotary instruments and irrigation solution for the purpose of the removal of residual Ca(OH)2 from root canals could be a future topic of study.
Conclusions
Within the limitations of this study, none of the irrigation solutions and ultrasonic activation of the irrigation solutions completely removed the Ca(OH)2 medicament from simulated immature root canals.
Declaration of interest
The authors deny any conflicts of interest. Authors affirm that they have no financial affiliation (e.g. employment, direct payment, stock holdings, retainers, consultantships, patent licensing arrangements or honoraria), or involvement with any commercial organization with direct financial interest in the subject or materials discussed in this manuscript, nor have any such arrangements existed in the past three years.
References
- 1. Kontakiotis E, Nakou M, Georgopoulou M. In vitro study of the indirect action of calcium hydroxide on the anaerobic flora of the root canal. Int Endod J. 1995;28:285–289 [DOI] [PubMed] [Google Scholar]
- 2. Zehnder M, Grawehr M, Hasselgren G, Waltimo T. Tissue-dissolution capacity and dentin-disinfecting potential of calcium hydroxide mixed with irrigating solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:608–613 [DOI] [PubMed] [Google Scholar]
- 3. Dominguez Reyes A, Munoz Munoz L, Aznar Martin T. Study of calcium hydroxide apexification in 26 young permanent incisors. Dent Traumatol. 2005;21:141–145 [DOI] [PubMed] [Google Scholar]
- 4. Walia T, Chawla HS, Gauba K. Management of wide open apices in non-vital permanent teeth with Ca(OH)2 paste. J Clin Pediatr Dent. 2000;25:51–56 [DOI] [PubMed] [Google Scholar]
- 5. Finucane D, Kinirons MJ. Non-vital immature permanent incisors: factors that may influence treatment outcome. Endod Dent Traumatol. 1999;15:273–277 [DOI] [PubMed] [Google Scholar]
- 6. Narita H, Itoh S, Imazato S, Yoshitake F, Ebisu S. An explanation of the mineralization mechanism in osteoblasts induced by calcium hydroxide. Acta Biomater. 2010;6:586–590 [DOI] [PubMed] [Google Scholar]
- 7. Calt S, Serper A, Ozcelik B, Dalat MD. pH changes and calcium ion diffusion from calcium hydroxide dressing materials through root dentin. J Endod. 1999;25:329–331 [DOI] [PubMed] [Google Scholar]
- 8. Windley W III Ritter A, Trope M. The effect of short-term calcium hydroxide treatment on dentin bond strengths to composite resin. Dent Traumatol. 2003;19:79–84 [DOI] [PubMed] [Google Scholar]
- 9. Erdemir A, Ari H, Gungunes H, Belli S. Effect of medications for root canal treatment on bonding to root canal dentin. J Endod. 2004;30:113–116 [DOI] [PubMed] [Google Scholar]
- 10. Guiotti FA, Kuga MC, Duarte MA, Sant'Anna AJ, Faria G. Effect of calcium hydroxide dressing on push-out bond strength of endodontic sealers to root canal dentin. Braz Oral Res. 2014;28: doi: 10.1590/S1806-83242014.50000002 [DOI] [PubMed] [Google Scholar]
- 11. Hosoya N, Kurayama H, Iino F, Arai T. Effects of calcium hydroxide on physical and sealing properties of canal sealers. Int Endod J. 2004;37:178–184 [DOI] [PubMed] [Google Scholar]
- 12. Calt S, Serper A. Dentinal tubule penetration of root canal sealers after root canal dressing with calcium hydroxide. J Endod. 1999;25:431–433 [DOI] [PubMed] [Google Scholar]
- 13. Kim SK, Kim YO. Influence of calcium hydroxide intracanal medication on apical seal. Int Endod J. 2002;35:623–628 [DOI] [PubMed] [Google Scholar]
- 14. Margelos J, Eliades G, Verdelis C, Palaghias G. Interaction of calcium hydroxide with zinc oxide-eugenol type sealers: a potential clinical problem. J Endod. 1997;23:43–48 [DOI] [PubMed] [Google Scholar]
- 15. Lambrianidis T, Kosti E, Boutsioukis C, Mazinis M. Removal efficacy of various calcium hydroxide/chlorhexidine medicaments from the root canal. Int Endod J. 2006;39:55–61 [DOI] [PubMed] [Google Scholar]
- 16. Lambrianidis T, Margelos J, Beltes P. Removal efficiency of calcium hydroxide dressing from the root canal. J Endod. 1999;25:85–88 [DOI] [PubMed] [Google Scholar]
- 17. Salgado RJ, Moura-Netto C, Yamazaki AK, Cardoso LN, de Moura AA, Prokopowitsch I. Comparison of different irrigants on calcium hydroxide medication removal: microscopic cleanliness evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:580–584 [DOI] [PubMed] [Google Scholar]
- 18. Lee SJ, Wu MK, Wesselink PR. The effectiveness of syringe irrigation and ultrasonics to remove debris from simulated irregularities within prepared root canal walls. Int Endod J. 2004;37:672–678 [DOI] [PubMed] [Google Scholar]
- 19. Balvedi RP, Versiani MA, Manna FF, Biffi JC. A comparison of two techniques for the removal of calcium hydroxide from root canals. Int Endod J. 2010;43:763–768 [DOI] [PubMed] [Google Scholar]
- 20. Nandini S, Velmurugan N, Kandaswamy D. Removal efficiency of calcium hydroxide intracanal medicament with two calcium chelators: volumetric analysis using spiral CT, an in vitro study. J Endod. 2006;32:1097–1101 [DOI] [PubMed] [Google Scholar]
- 21. Kenee DM, Allemang JD, Johnson JD, Hellstein J, Nichol BK. A quantitative assessment of efficacy of various calcium hydroxide removal techniques. J Endod. 2006;32:563–565 [DOI] [PubMed] [Google Scholar]
- 22. van der Sluis LW, Wu MK, Wesselink PR. The evaluation of removal of calcium hydroxide paste from an artificial standardized groove in the apical root canal using different irrigation methodologies. Int Endod J. 2007;40:52–57 [DOI] [PubMed] [Google Scholar]
- 23. Taşdemir T, Celik D, Er K, Yildirim T, Ceyhanli KT, Yesilyurt C. Efficacy of several techniques for the removal of calcium hydroxide medicament from root canals. Int Endod J. 2011;44:505–509 [DOI] [PubMed] [Google Scholar]
- 24. Rodig T, Vogel S, Zapf A, Hulsmann M. Efficacy of different irrigants in the removal of calcium hydroxide from root canals. Int Endod J. 2010;43:519–527 [DOI] [PubMed] [Google Scholar]
- 25. Gupta S, Jawanda MK, Sm M, Bharti A. Qualitative histological evaluation of hard and soft tissue components of human permanent teeth using various decalcifying agents – a comparative study. J Clin Diagn Res. 2014;8:ZC69–ZC72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rödig T, Hirschleb M, Zapf A, Hulsmann M. Comparison of ultrasonic irrigation and RinsEndo for the removal of calcium hydroxide and Ledermix paste from root canals. Int Endod J. 2011;44:1155–1161 [DOI] [PubMed] [Google Scholar]
- 27. Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zaia AA, Valdrighi L, Souza-Filho FJ. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36:267–275 [DOI] [PubMed] [Google Scholar]
- 28. Ram Z. Effectiveness of root canal irrigation. Oral Surg Oral Med Oral Pathol. 1977;44:306–312 [DOI] [PubMed] [Google Scholar]
- 29. Abou-Rass M, Piccinino MV. The effectiveness of four clinical irrigation methods on the removal of root canal debris. Oral Surg Oral Med Oral Pathol. 1982;54:323–328 [DOI] [PubMed] [Google Scholar]
- 30. Huang TY, Gulabivala K, Ng YL. A bio-molecular film ex-vivo model to evaluate the influence of canal dimensions and irrigation variables on the efficacy of irrigation. Int Endod J. 2008;41:60–71 [DOI] [PubMed] [Google Scholar]
- 31. De Moor RJ, Meire M, Goharkhay K, Moritz A, Vanobbergen J. Efficacy of ultrasonic versus laser-activated irrigation to remove artificially placed dentin debris plugs. J Endod. 2010;36:1580–1583 [DOI] [PubMed] [Google Scholar]
- 32. Moon YM, Kim HC, Bae KS, Baek SH, Shon WJ, Lee W. Effect of laser-activated irrigation of 1320-nanometer Nd: YAG laser on sealer penetration in curved root canals. J Endod. 2012;38:531–535 [DOI] [PubMed] [Google Scholar]
- 33. Arslan H, Capar ID, Saygili G, Gok T, Akcay M. Effect of photon-initiated photoacoustic streaming on removal of apically placed dentinal debris. Int Endod J. 2014;47:1072–1077 [DOI] [PubMed] [Google Scholar]
- 34. Capar ID, Ozcan E, Arslan H, Ertas H, Aydinbelge HA. Effect of different final irrigation methods on the removal of calcium hydroxide from an artificial standardized groove in the apical third of root canals. J Endod. 2014;40:451–454 [DOI] [PubMed] [Google Scholar]
- 35. Kuga MC, Tanomaru-Filho M, Faria G, So MV, Galletti T, Bavello JR. Calcium hydroxide intracanal dressing removal with different rotary instruments and irrigating solutions: a scanning electron microscopy study. Braz Dent J. 2010;21:310–314 [DOI] [PubMed] [Google Scholar]