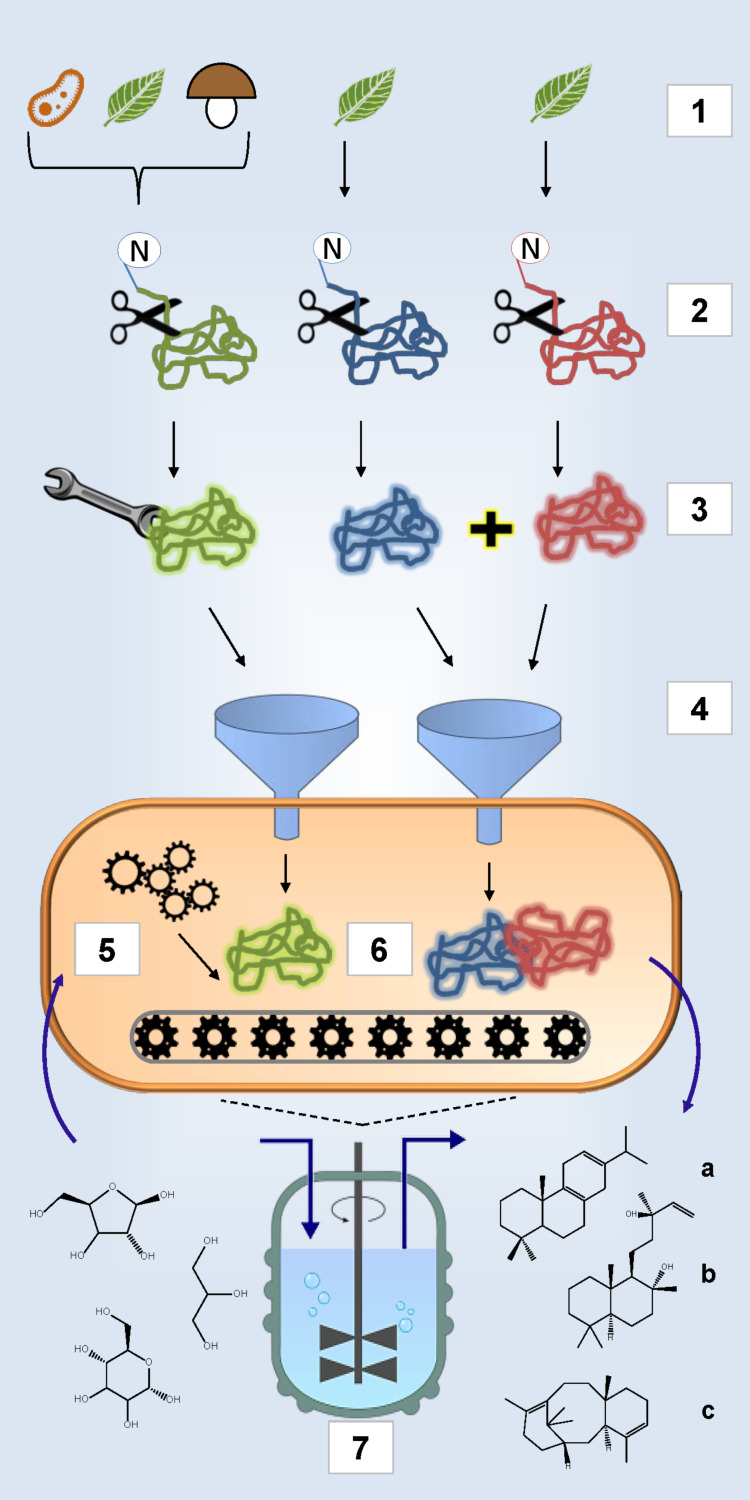
Figure 1.
Implementation of a microbial cell factory. 1: Selection of enzymes from different species. P450 and related reductase enzymes (indicated with blue and red knots respectively) derive almost exclusively from plants; terpene synthases and other enzymes that are involved in precursor formation (indicated with green knot) can be obtained from various organisms (indicated through symbolic bacteria, leaf (representing plants in general) and fungi). 2: Eukaryotic enzymes have to be engineered for functional and soluble expression in prokaryotic hosts like E. coli, Removal of the N-terminal and thereby the cell-wall-localization domain (indicated through scissors) is a standard procedure in engineering plant enzymes; 3: Further engineering steps are not mandatory but often entail site-directed mutagenesis (indicated through wrench) of TPS (green) for product modulation or introduction of a linker-coding sequence for co-expression of P450 monooxygenase and reductase (blue and red); 4: Heterologous expression in E. coli (depicted in orange). Construction of synthetic operons and screening for highest yield of plasmid systems generally precedes genomic integration; 5: Isoprenoid precursor supply: precursor flux has to be balanced carefully to avoid metabolic overload and accumulation of unwanted byproducts; 6: Downstream terpenoid biosynthesis using heterologous enzymes; 7: Upscaling of terpene production in fermentation systems using different carbon sources (left) for optimally engineered E. coli strains is a potential future source for valuable diterpenes like miltiradiene (a), sclareol (b) or taxadiene (c).