Figure 3.
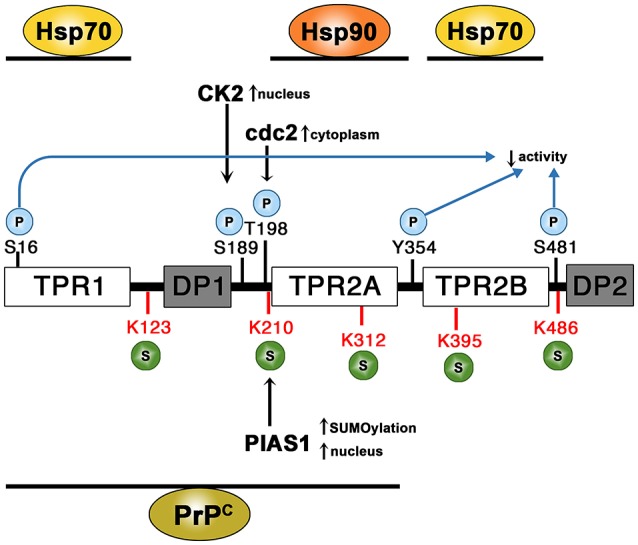
Domain structure of STI1 and sites of post-translational modifications (PTM). STI1 is composed of three structurally similar tetratricopeptide repeat domains (TPR1, TPR2A, and TPR2B) and two regions rich in aspartate and proline residues (DP1 and DP2). The protein is subject to phosphorylation (S16, S189, T198, Y354, and S481). CK2 phosphorylation at S189 induces STI1 accumulation in the nucleus. In contrast phosphorylation by cdc2 at T198 localizes STI1 to the cytoplasm. Five possible SUMOylation sites have been identified (K123, K210, K312, K395, and K486). SUMOylation by PIAS1 at K210 may stimulate SUMOylation at the alternate sites. Association of PIAS1 with STI1 by a SUMO-independent mechanism increases STI1 nuclear accumulation. Regions that bind HSP70, HSP90, and PrPC are illustrated.
