Abstract
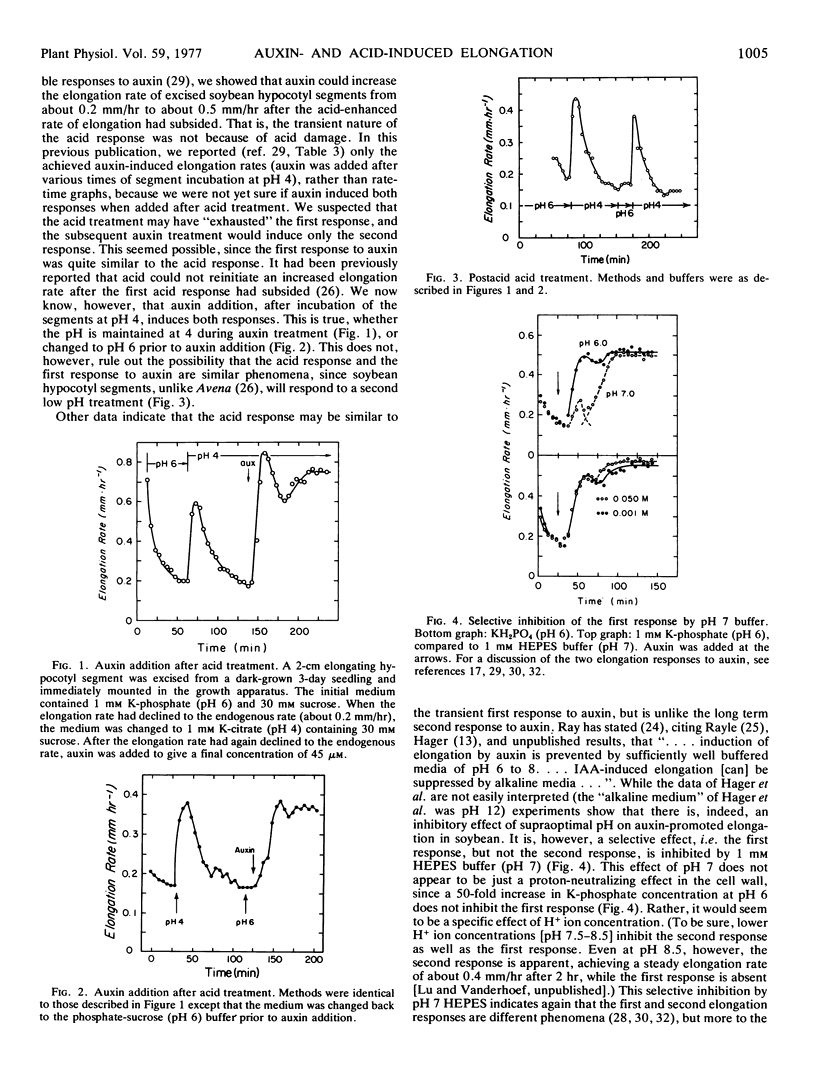
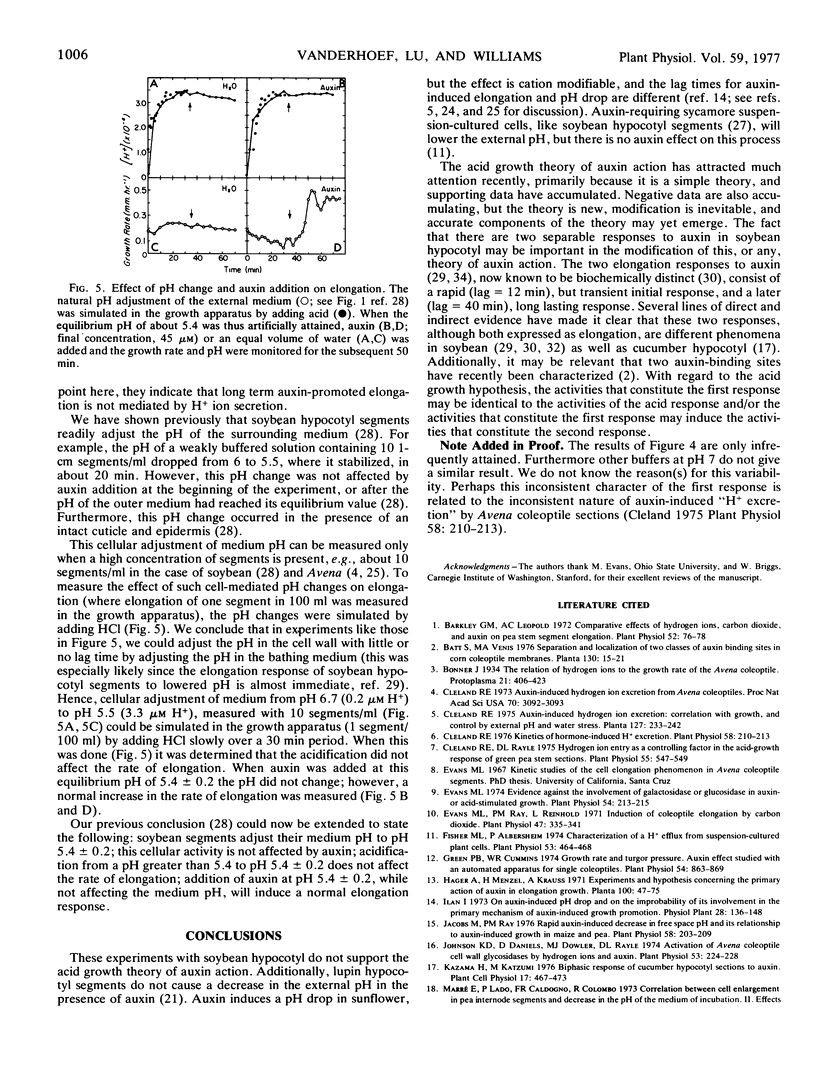
Acid-induced growth was compared to auxin-induced growth. After a transient pH 4-induced increase in the elongation rate was completed, auxin could still induce an enhanced rate of elongation in soybean (Glycine max) hypocotyl segments. This auxin response occurred both when the medium was changed to pH 6 before auxin addition, and when the auxin was added directly to the pH 4 medium. This postacid response to auxin was persistent, and quite unlike a postacid response to acid, which was again shortlived. One mm N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7) inhibited the first response to auxin (the first response to auxin being similar to the acid response), but not the second response. This did not appear to be simply a hydrogen ion neutralizing effect, however, since a 50-fold increase in buffer concentration at pH 6 did not inhibit the first response. Decrease in the pH of the external medium, previously shown to occur with excised soybean hypocotyl segments, was not affected by auxin. Furthermore, this pH drop, during which the cells appear to be adjusting their external pH to about 5.4, did not result in an increased rate of elongation. Addition of auxin after the equilibrium pH had been attained did not alter the pH, but it did increase the rate of elongation, eliciting a normal auxin response. It was concluded that hydrogen ions do not mediate in long term auxin-induced elongation in soybean hypocotyl.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkley G. M., Leopold A. C. Comparative effects of hydrogen ions, carbon dioxide, and auxin on pea stem segment elongation. Plant Physiol. 1973 Jul;52(1):76–78. doi: 10.1104/pp.52.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. Hydrogen Ion Entry as a Controlling Factor in the Acid-growth Response of Green Pea Stem Sections. Plant Physiol. 1975 Mar;55(3):547–549. doi: 10.1104/pp.55.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Auxin-induced hydrogen ion excretion from Avena coleoptiles. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3092–3093. doi: 10.1073/pnas.70.11.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L. Evidence Against the Involvement of Galactosidase or Glucosidase in Auxin- or Acid-stimulated Growth. Plant Physiol. 1974 Aug;54(2):213–215. doi: 10.1104/pp.54.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M., Reinhold L. Induction of coleoptile elongation by carbon dioxide. Plant Physiol. 1971 Mar;47(3):335–341. doi: 10.1104/pp.47.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. L., Albersheim P. Characterization of a H Efflux from Suspension-cultured Plant Cells. Plant Physiol. 1974 Mar;53(3):464–468. doi: 10.1104/pp.53.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. B., Cummins W. R. Growth rate and turgor pressure: auxin effect studies with an automated apparatus for single coleoptiles. Plant Physiol. 1974 Dec;54(6):863–869. doi: 10.1104/pp.54.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Rapid Auxin-induced Decrease in Free Space pH and Its Relationship to Auxin-induced Growth in Maize and Pea. Plant Physiol. 1976 Aug;58(2):203–209. doi: 10.1104/pp.58.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Daniels D., Dowler M. J., Rayle D. L. Activation of Avena coleoptile cell wall glycosidases by hydrogen ions and auxin. Plant Physiol. 1974 Feb;53(2):224–228. doi: 10.1104/pp.53.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch J. P., Nitsch C. Studies on the Growth of Coleoptile and First Internode Sections. A New, Sensitive, Straight-Growth Test for Auxins. Plant Physiol. 1956 Mar;31(2):94–111. doi: 10.1104/pp.31.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970 Aug;46(2):250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Findley J. S., Burke J. J., Blizzard W. E. Auxin Has No Effect on Modification of External pH by Soybean Hypocotyl Cells. Plant Physiol. 1977 May;59(5):1000–1003. doi: 10.1104/pp.59.5.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A., Lu T. Y. Two elongation responses to auxin respond differently to protein synthesis inhibition. Plant Physiol. 1976 Sep;58(3):402–404. doi: 10.1104/pp.58.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A. Separation of two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci U S A. 1975 May;72(5):1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A., Williams C. A., Brinkmann K. A. Additional evidence for separable responses to auxin in soybean hypocotyl. Plant Physiol. 1976 May;57(5):817–819. doi: 10.1104/pp.57.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]


