Fig. 2. EAAT2-D504N inhibits caspase-3 cleavage and CTE-SUMO1 formation.
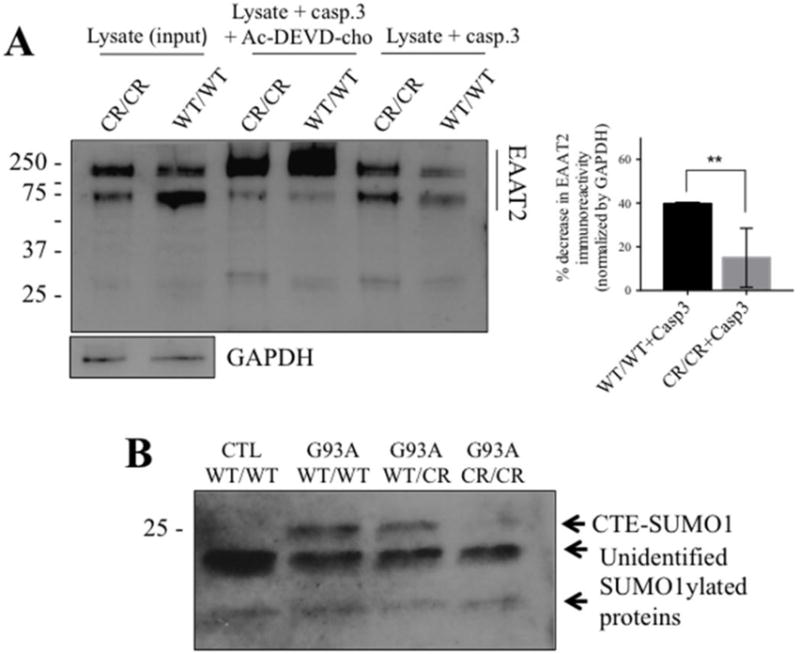
(A) Recombinant active caspase-3 cleaves EAAT2WT/WT in spinal cord homogenates but cleavage is inhibited in spinal cord homogenates of EAAT2CR/CR mice (custom ABR518-536 at 1:10,000). One blot is shown to represent the effect of caspase-3 cleavage on EAAT2 immunoreactivity in the presence or absence of caspase-3 inhibitor together with quantification of the effect. EAAT2 content is normalized to GAPDH immunoreactivity in the input lysate samples. Cleavage was quantified as disappearance of EAAT2-WT or EAAT2-CR monomer and oligomer immunoreactivity in the presence of active caspase-3 from 3 different cleavage reactions. ** p<0.005, two-tailed t-test. (B) CTE-SUMO1 is seen at ~25 kDa in spinal cord homogenates of SOD1-G93A mice (G93A) expressing EAAT2WT/WT or EAAT2WT/CR, but significantly decreased in SOD1-G93A mice expressing EAAT2CR/CR (Cell Signaling #4930 anti-SUMO1 at 1:250). It is also not seen in spinal cord homogenates of control non-ALS EAAT2WT/WT control mice. Two unidentified lower molecular weight SUMO1 immunopositive bands are shown as evidence of equal protein loading among the different lanes of the blot.
