Figure 1.
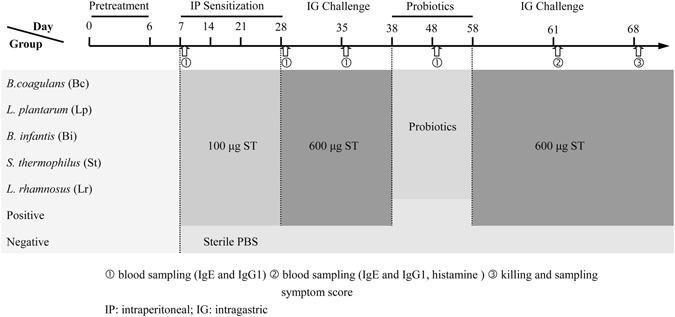
Experimental design of in vivo sensitization and probiotic treatment. Groups of mice (n = 8) were immunized by intraperitoneal (IP) injection of 100 μg purified ST/mouse on days 7, 14, 21 and 28, then challenged by receiving one intragastric gavage (IG) dose of ST (600 μg ST/mouse) on day 35. From day 38 to 58, sensitized mice were orally administered with different live probiotic bacteria daily. On day 61, mice were re-challenged by IG administration of ST. Blood samples were collected from the retro-orbital plexus on days 7, 28, 35, 48 and 61, and the appearance of symptoms of systemic anaphylaxis was observed on day 61. On day 68, mice were killed to collect blood, spleen, MLN and intestinal tissues. Negative control animals received equal amounts of sterile PBS at each sensitization and challenge point. Positive control received probiotic-free sterile PBS during bacteria treatment periods. In a separate experiment, three groups of mice (n = 8) including Bc-treated group, positive and negative controls were immunized following the same procedure as described above. Mice were sacrificed to collect spleen and intestinal tissues for immunoblotting, mTOR ELISA and immunohistochemical analysis.
