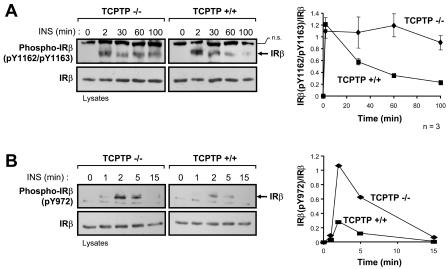
FIG. 8.
Phosphorylation of the IRβ on Y972 is enhanced, whereas Y1162/Y1163 phosphorylation is prolonged in TCPTP-null cells. TCPTP−/− and TCPTP+/+ fibroblasts were deprived of serum and stimulated with 10 nM insulin (INS) as indicated. Cells were lysed in NP-40 buffer and clarified by centrifugation, and proteins were resolved by SDS-PAGE and immunoblotted with antibodies to the phosphorylated IRβ. (A) Immunoblots of cell lysates were probed with antibodies specific for the IRβ Y1162/1163 phosphorylation site [Phospho-IRβ(pY1162/pY1163)] and then stripped and reprobed for IRβ. IRβ is indicated by an arrow in the phosphorylation immunoblot. A nonspecific protein (n.s.) recognized by the phosphorylation antibody is also shown and confirms equal protein loading. The graph on the right-hand side shows Phospho-IRβ(pY1162/pY1163) immunoblots quantitated by densitometric analysis and normalized for total IRβ protein in the corresponding IRβ immunoblots, with the Phospho-IRβ(pY1162/pY1163)/IRβ ratio in the absence of insulin being set at zero. Units shown are arbitrary and are the means ± standard errors of three independent experiments. (B) Immunoblots were probed with antibodies specific for the IRβ Y972 phosphorylation site [Phospho-IRβ(pY972)] and then stripped and reprobed with IRβ antibodies. Similar results were observed in two independent experiments. IRβ in the phosphorylation immunoblot is indicated by an arrow. A nonspecific protein (n.s.) recognized by the phosphorylation antibody is also indicated and confirms equal protein loading. The graph at the right-hand side shows the Phospho-IRβ(pY972) immunoblot quantitated by densitometric analysis and normalized for total IRβ protein, with the Phospho-IRβ(pY972)/IRβ ratio in the absence of insulin being set at zero. Units shown are arbitrary.