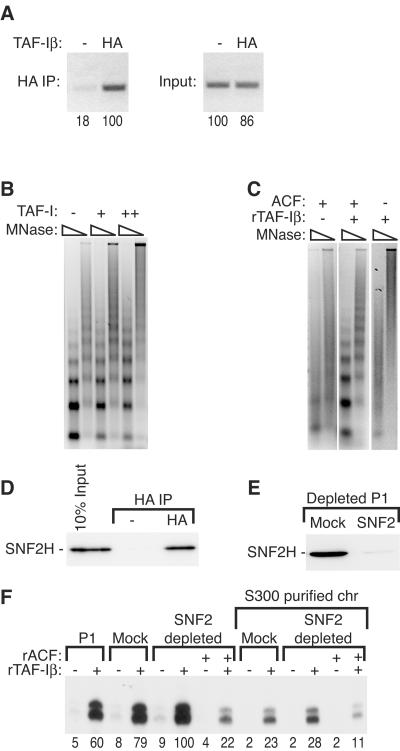
FIG. 7.
TAF-I interacts with chromatin and chromatin-remodeling factors. (A) Transcription reaction mixtures containing HA-tagged or untagged TAF-Iβ were incubated for 30 min and then subjected to chromatin immunoprecipitation (IP) in vitro with HA antibody. Coimmunoprecipitated DNA was analyzed by semiquantitative PCR and 1% agarose gel electrophoresis. (B) Preassembled chromatin was incubated with buffer alone (−) or 15.3 (+) or 53 (++) ng of HeLa TAF-I from the peak Superdex 200 fractions per μl (Fig. 4B) and then subjected to limited micrococcal nuclease (MNase) digestion. A 15.3-ng/μl concentration of this fraction is roughly equivalent to 4 μl of fractions 25 and 26 assayed in Fig. 4B. (C) Chromatin assembly reaction mixtures were prepared without rNAP-1. Where indicated, ACF and 8 μM rTAF-Iβ were present during assembly. After assembly, the chromatin was subjected to limited micrococcal nuclease digestion. (D) HA-tagged or untagged TAF-Iβ (58 pmol) was incubated with 36 μg of the PC1 fraction for 30 min and subsequently immunoprecipitated with HA antibody. The immunoprecipitates and input (10%) were analyzed by immunoblot assay with an anti-SNF2 antibody. (E) The mock-depleted or anti-SNF2-depleted PC1 fraction was analyzed by immunoblotting with the anti-SNF2 antibody. (F) Transcription reaction mixtures were prepared with 3 μg of PC1, mock-depleted PC1, or anti-SNF2-depleted PC1 on unpurified and S300 spin column-purified chromatin (chr). rACF (170 nM) and rTAF-Iβ (3 μM) were added where indicated. The relative level of transcription was quantified by Phosphorimager, with the maximal level defined as 100 U, and is indicated below each lane.