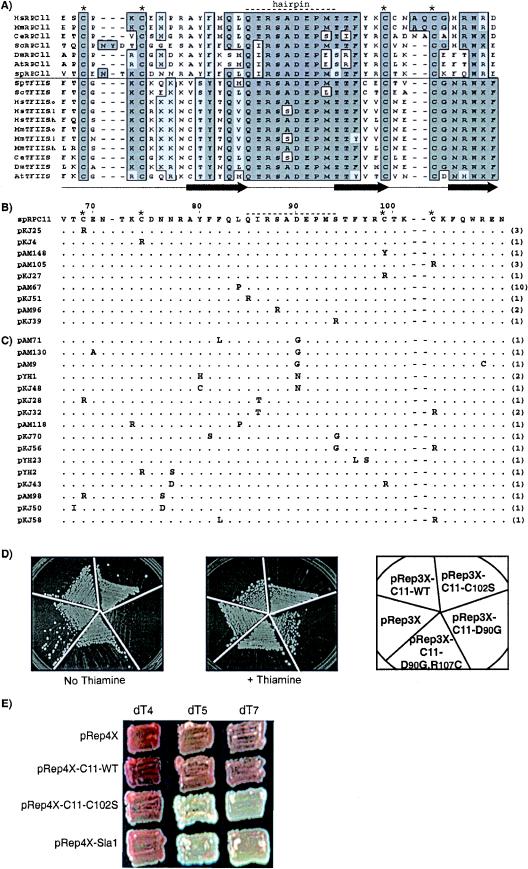
FIG.1.
S. pombe rpc11 mutants that increase tRNA-mediated suppression occur in invariant residues in the Zn ribbon and are predicted to decrease RNA 3′ cleavage activity. (A) Amino acid alignment of domain III of Rpc11p and the zinc ribbon domains of TFIIS of multiple species, above and below the horizontal line, respectively. The positions of invariant cysteines and a stretch of residues that corresponds to the acidic hairpin of TFIIS are indicated above by asterisks and a dashed line, respectively. Conserved residues are boxed and shaded. Filled arrows below the alignment represent beta strands 1 to 3 of TFIIS (29). (B) All Rpc11p mutants that contained single substitutions in domain III; the numbers of independent isolates are in parentheses. Wild-type S. pombe Rpc11p is shown in the first line, numbered above (8). (C) Mutants with two substitutions in domain III as their only mutations. (D) Effects of selected Rpc11p constructs on growth in nonlimiting adenine in which expression is on (No Thiamine) or repressed (+ Thiamine). (E) Suppression by C102S, C11-WT, Sla1, and pRep4X in sla1+ strains whose tRNASerUGAM gene terminators are comprised of dT4, dT5, and dT7 (yAMm4T-2, yYHm5T, and yYH1, respectively), as indicated.