Figure 1. Li+ increases synaptic GluA2 and its associated scaffolding protein in cultured hippocampal neurons.
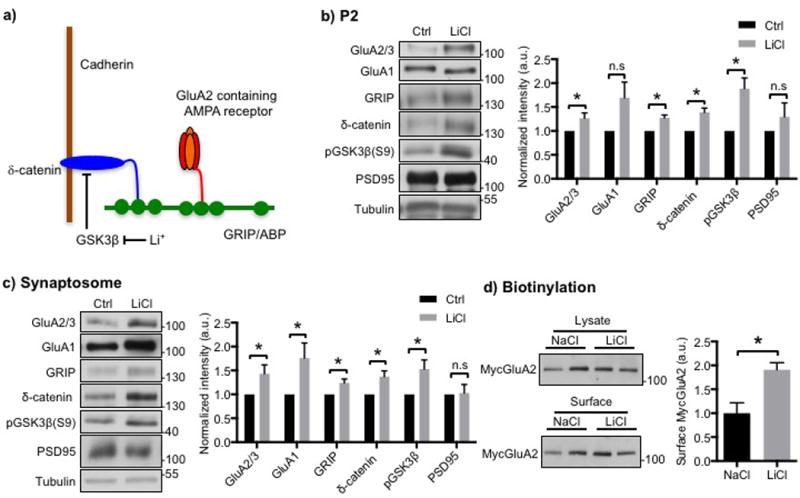
a) Model for the regulation of synaptic GluA2 by Li+. The GluA2 AMPA receptor subunit is anchored at the synaptic membrane by an interaction of its C-terminus with the fifth PDZ domain of the scaffolding protein, GRIP, and the related ABP protein. The second PDZ domain of GRIP associates with the C-terminus of δ-catenin. δ-catenin binds to the juxtamembrane region of cadherin, which is a synaptic cell adhesion protein. Phosphorylation of δ-catenin by GSK3 leads to the degradation of δ-catenin, which disrupts the δ-catenin-GRIP scaffold at the synapse and lowers synaptic levels of GluA2. Inhibition of GSK3 by Li+ stabilizes δ-catenin, which elevates GRIP and GluA2 at the synapse. See Silverman et al., (2007) and the text for details. Representative immunoblots and quantitative analysis of b) P2 membrane fraction and c) synaptosome fraction from cultured hippocampal neurons treated with NaCl (control) or LiCl showing that LiCl treatment increased GluA2/3, GRIP, and δ-catenin levels (n=5 and 10 experiments in b) and c), respectively, *p<0.05 and **p<0.01, unpaired two-tailed Student’s t tests). GSK3β inhibition by LiCl was identified by an increase in its phosphorylation. d) Representative immunoblots of surface biotinylation and a summary graph for cultured hippocampal neurons treated with NaCl or LiCl showing that LiCl treatment increased surface GluA2/3 levels (n=4 experiments, *p<0.05, unpaired two-tailed Student’s t tests).
