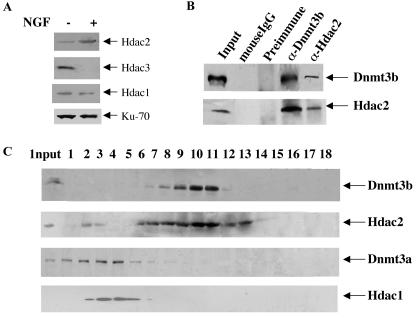
FIG. 8.
Association of Dnmt3b with Hdac2 in PC12 cells. (A) Up-regulation of Hdac2 in PC12 cells after differentiation. Whole-cell extracts (200 μg of protein) from untreated (−) and NGF-treated (+) cells (6 days) were used for Western blot analysis with Hdac2 antibody. The blot was stripped and reprobed with Hdac3 or Hdac1 antibody. Hdac1 could not be detected with a regular ECL Western blotting detection system (Amersham). A low level of Hdac1 was detected using highly sensitive chemiluminescent peroxidase substrate from Sigma. (B) Coimmunoprecipitation analysis. Whole-cell extracts (1 mg of protein) from PC12 cells treated with NGF for 6 days were immunoprecipitated with anti-Dnmt3b (α-Dnmt3b) or anti-Hdac2 (α-Hdac2) antibody. Dnmt3b preimmune serum and mouse IgG were used as respective negative controls. The precipitated proteins were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and subjected to Western blot analysis with Dnmt3b and Hdac2 antibodies. (C) Cosedimentation of Dnmt3b with a population of Hdac2 during glycerol density gradient fractionation. Whole-cell extract from 107 PC12 cells (NGF treated for 6 days) was fractionated by glycerol density (5 to 30%) gradient. Fractions were collected from the bottom of the tube and separated on an SDS-PAGE gel, followed by Western blot analysis with Dnmt3a, Dnmt3b, and Hdac1 to -3 antibodies.