Summary
Randomized, controlled trials have demonstrated that procalcitonin (PCT)-guided algorithms for antibiotic therapy may decrease antibiotic use. However, PCT use in a real-world setting was not associated with decreased antibiotic days, incidence of Clostridium difficile infection, or mortality.
Keywords: critical care, outcome assessment, antibacterial agents/administration and dosage, Clostridium difficile.
Abstract
Background.
Randomized trials support use of procalcitonin (PCT)-based algorithms to decrease duration of antibiotics for critically ill patients with sepsis. However, current use of PCT and associated outcomes in real-world clinical settings is unclear. We sought to determine PCT use in critically ill patients with sepsis in the United States and to examine associations between PCT use and clinical outcomes.
Methods.
This was a retrospective cohort study of approximately 20% of patients with sepsis hospitalized in US intensive care units. Hierarchical regression models were used to determine associations of PCT use with outcomes (antibiotic-days, incidence of Clostridium difficile infection, and in-hospital mortality). Sensitivity analyses were conducted to assess robustness of findings to different methods used to address unmeasured confounding (eg, instrumental variable, difference-in-differences analyses).
Results.
Among 20750 critically ill patients with sepsis in 107 hospitals with PCT available, 3769 (18%) patients had PCT levels checked; 1119 (29.7%) had serial PCT measurements. PCT use was associated with increased antibiotic-days (adjusted relative risk, 1.1; 95% confidence interval [CI], 1.15–1.18) and incidence of C. difficile (adjusted odds ratio, 1.42; 95% CI, 1.09–1.85) without a change in mortality (adjusted hazard ratio, 1.05; 95% CI, 0.93–1.19). Analysis of PCT use by instrumental variable and difference-in-difference analyses showed similar lack of antibiotic or outcome improvements associated with PCT use.
Conclusions.
PCT use was not associated with improved antibiotic use or other clinical outcomes in real-world settings. Programs to improve implementation of PCT-based strategies are warranted prior to widespread adoption.
Sepsis affects approximately 1 million adults in the United Stated [1], with rising incidence and 15%–20% in-hospital case-fatality rates [2]. Decisions regarding antibiotic cessation are critical to patient outcomes during sepsis. Courses of antibiotics that are too long may produce adverse events such as Clostridium difficile colitis and antibiotic resistance [3]. Because approximately 1 in 3 hospitalized patients may inappropriately receive antibiotics [4], better strategies are needed to guide clinical decision-making regarding initiation and cessation of antibiotics for patients with sepsis.
Procalcitonin (PCT) has emerged as a biomarker of bacterial infection that may inform clinical decisions regarding early cessation of antibiotics. Randomized trials in patients with lower respiratory tract infection [5–7] and in critically ill patients with sepsis from undifferentiated infection [8–13] have demonstrated efficacy of PCT for reducing antibiotic duration, without increasing morbidity or mortality. Although PCT looks promising in the clinical trial setting, little is known about how PCT has been used in real-world practice in the United States or if use of PCT outside of experimental trials is associated with decreased antibiotic use or antibiotic-associated complications. Thus, recent guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America give only a weak recommendation to use serial PCT measurements as a guide to decrease antibiotic use [14].
We sought to explore variation in use patterns for PCT during sepsis in US intensive care units (ICUs). Leveraging natural variation in PCT uptake, we analyzed antibiotic duration and patient outcomes associated with PCT use. Based on prior randomized, controlled trial results, we hypothesized that use of PCT would be associated with a reduction in antibiotic duration and a decrease in antibiotic-associated adverse outcomes, without a significant change in mortality. Some study results have been previously reported in the form of an abstract [15, 16].
METHODS
Sepsis Cohort
We performed a retrospective cohort study using the Premier database (Charlotte, North Carolina), which includes approximately 20% of hospitalized patients in nonfederal US hospitals. Premier data include date-stamped pharmacy, laboratory, and diagnostics billing information. Patients hospitalized in an ICU with sepsis were identified using high-positive predictive value (>90%) explicit sepsis International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), codes present on admission [17] in conjunction with receipt of an antibiotic on their first hospital day and an ICU charge (see Supplementary Table 1 for a full list of ICD-9-CM codes used). Because randomized trials do not support the efficacy of PCT for guiding decisions to initiate antibiotics in the intensive care setting [14, 18], we excluded patients who did not receive an antibiotic. We also excluded patients who were in a hospital with fewer than 25 sepsis cases in 2012 or were in a hospital that did not have a PCT order in 2012.
Procalcitonin Use and Covariates
We identified PCT use from laboratory billing files and determined temporal relationships between PCT orders and the timing of the initial antibiotic dose. We recorded patient demographics, hospital characteristics, and attending physician specialty. For each patient, we obtained information on comorbid conditions [19–21], acute organ failures that were present on admission [22, 23], and site of infection [24] using previously validated ICD-9-CM algorithms.
Outcomes
In our primary analysis, we sought to identify the association between PCT use and antibiotic days of therapy (DOT) during hospitalization, including use during and following intensive care. Because PCT levels signal whether bacterial infection is likely and whether antibiotics may be indicated, rather than which antibiotics may be indicated, multiple antibiotics ordered in 1 day were measured as 1 DOT [25]. Secondary outcomes of interest included rates of new-onset C. difficile infection as defined by ICD-9-CM codes that were not present on admission (sensitivity 78%, specificity 99.7%) [26, 27] and in-hospital mortality.
Statistical Analyses
We reported continuous variables using means and standard deviations or median and interquartile range depending on the distribution and categorical variables as percentages. We analyzed associations between PCT measurement and patient and hospital characteristics and calculated individual hospital risk-adjusted PCT rates (defined as number of patients who had PCT checked/number of sepsis cases) using hierarchical regression models, with each hospital serving as a random intercept. Because of the likelihood that PCT testing was unavailable at hospitals that had not used PCT, we restricted the analysis of factors associated with PCT use to hospitals that used PCT at least once. We estimated the proportion of variability in PCT use explained by patient and hospital characteristics by calculating intraclass correlation coefficients from hierarchical regression models [28].
We used hierarchical Poisson regression models to analyze associations between PCT use and antibiotic DOT, and we used hierarchical logistic regression for modeling C. difficile infection. In order to account for potential “immortal time” prior to PCT orders, we used a time-varying specification of PCT orders in Cox proportional hazards models, assessing associations between PCT orders and patient in-hospital mortality [29]. Immortal time was defined as the time prior to a PCT order (or prior to a second PCT order in analyses of serial orders) during which patients with PCT orders were necessarily alive (immortal) but patients who did not have PCT orders may have died. Models were adjusted for patient demographics, patient comorbidities, acute organ failures present on admission, site of infection, hospital characteristics and attending physician specialty, patient comorbidities, and acute organ failures (see details of covariates in Table 1). Length of stay was included in models of antibiotic use but not in models of mortality or C. difficile infection.
Table 1.
Baseline Patient Characteristics Associated With Procalcitonin Use
| Characteristic | No Procalcitonin (N = 16981) | Procalcitonin (N = 3769) | Multivariable- Adjusted Odds Ratio |
|---|---|---|---|
| Age, y | 65.9 (16.2) | 65.5 (16.1) | 0.99 (0.99–1.00) |
| Male | 8393 (49.4%) | 1910 (50.7%) | 0.95 (0.87–1.04) |
| Race | |||
| White | 12625 (74.4%) | 2751 (73.0%) | Reference |
| Black | 2010 (11.8%) | 446 (11.8%) | 0.95 (0.87–1.04) |
| Other | 2346 (13.8%) | 572 (15.2%) | 0.99 (0.85–1.16) |
| Geographic location | |||
| Northeast | 1306 (7.7%) | 87 (2.3%) | Reference |
| Midwest | 4135 (24.4%) | 782 (20.8%) | 2.23 (0.44–11.29) |
| South | 9385 (55.3%) | 1848 (49.0%) | 2.38 (0.51–11.29) |
| West | 2155 (12.7%) | 1052 (27.9%) | 6.20 (1.00–38.35) |
| Teaching hospital | 6419 (37.8%) | 1204 (31.9%) | 0.59 (0.25–1.41) |
| Attending specialty | |||
| Internal medicine | 13907 (83.1%) | 3143 (83.8%) | Reference |
| Surgical | 1016 (6.1%) | 179 (4.8%) | 0.69 (0.56–0.86) |
| Pulmonary/Critical care |
1622 (9.7%) | 384 (10.2%) | 1.12 (0.94–1.33) |
| Cardiology | 192 (1.2%) | 45 (1.2%) | 0.96 (0.63–1.47) |
| Comorbidities | |||
| Heart failure | 4613 (27.2%) | 1140 (30.3%) | 1.16 (1.05–1.30) |
| Diabetes | 6402 (37.7%) | 1368 (36.3%) | 0.99 (0.90–1.09) |
| Hypertension | 10650 (62.7%) | 2299 (61.0%) | 1.07 (0.96–1.18) |
| Stroke/Transient ischemic attack | 284 (1.7%) | 68 (1.8%) | 1.08 (0.76–1.53) |
| Coronary artery disease/Myocardial infarction | 4609 (27.1%) | 945 (25.1%) | 1.10 (0.98–1.23) |
| Chronic kidney disease | 4989 (29.4%) | 1060 (28.1%) | 0.93 (0.82–1.04) |
| Chronic obstructive pulmonary disease | 5880 (34.6%) | 1260 (33.4%) | 0.97 (0.88–1.07) |
| Valvular heart disease | 1436 (8.5%) | 357 (9.4%) | 1.14 (0.97–1.34) |
| Peripheral vascular disease | 1933 (11.4%) | 373 (9.9%) | 0.85 (0.72–0.99) |
| Cancer | 2382 (14.0%) | 477 (12.7%) | 0.92 (0.81–1.06) |
| Cirrhosis | 1121 (6.6%) | 271 (7.2%) | 1.02 (0.85–1.24) |
| Dementia | 731 (4.3%) | 149 (4.0%) | 1.02 (0.81–1.28) |
| Infection site | |||
| Pneumonia | 5798 (34.1%) | 1364 (36.2%) | 1.32 (1.19–1.46) |
| Gastrointestinal | 2183 (12.9%) | 475 (12.6%) | 1.07 (0.93–1.24) |
| Urinary tract infection | 5693 (33.5%) | 1191 (31.6%) | 0.98 (0.89–1.09) |
| Skin/Soft tissue | 1539 (9.1%) | 339 (9.0%) | 0.96 (0.82–1.13) |
| Bacteremia | 216 (1.3%) | 48 (1.3%) | 1.19 (0.81–1.76) |
| Acute organ failures, present on admission | |||
| Respiratory | 6750 (39.8%) | 1624 (43.1%) | 1.22 (1.10–1.34) |
| Shock | 7910 (46.6%) | 1886 (50.0%) | 1.24 (1.13–1.37) |
| Renal | 9948 (58.6%) | 2264 (60.1%) | 1.05 (0.94–1.17) |
| Neurologic | 2645 (15.6%) | 635 (16.9%) | 1.02 (0.90–1.15) |
| Hematologic | 2932 (17.3%) | 734 (19.5%) | 1.09 (0.97–1.23) |
| Acidosis | 4710 (27.7%) | 1125 (29.9%) | 1.17 (1.06–1.30) |
| Hepatic | 930 (5.5%) | 244 (6.5%) | 1.21 (1.00–1.48) |
| Length of stay, days | 10.8 (12.4) | 12.0 (13.2) | 1.02 (1.01–1.02) |
Data presented as mean (standard deviation), N (%), or adjusted odds ratio (95% confidence interval).
Sensitivity Analyses
We performed multiple sensitivity analyses to explore the robustness of our primary analysis to different specifications of the cohort and various methods to attenuate unmeasured confounding. Details of sensitivity analyses are available in the Supplementary Materials. Briefly, we explored associations between PCT orders and antibiotic DOT in different specifications of the sepsis cohort, including analyses expanded to hospitals that did not use PCT, hospitals that used only PCT in the year prior, and hospitals that used serial PCT measurements. In addition to identifying different associations between PCT use and infection site, we conducted subgroup analysis by infection source. Finally, we conducted multiple ecological analyses to reduce confounding by indication for PCT testing, including a difference-in-differences approach for hospitals that did and did not adopt PCT testing over time.
We used SAS version 9.4 (Cary, North Carolina) for all analyses, with an alpha threshold of 0.05. The Boston University Medical Campus Institutional Review Board approved study procedures.
RESULTS
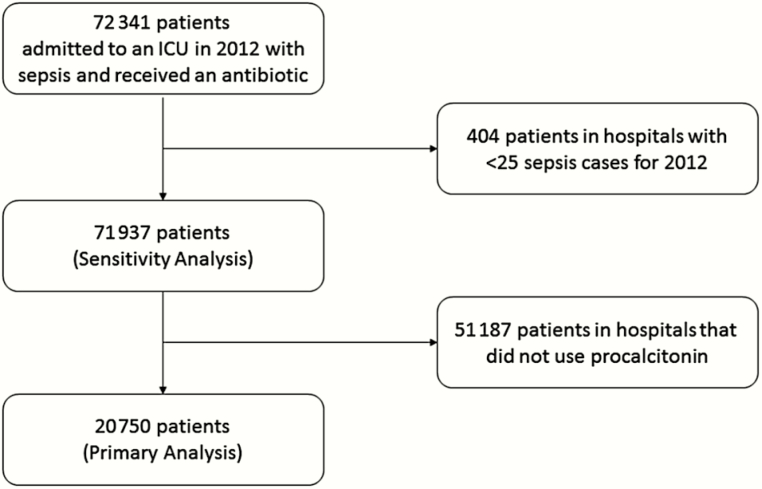
We identified 20750 patients in 107 hospitals that used PCT levels at least once (Figure 1), among whom 3769 (18%) had PCT measurements during a sepsis hospitalization. Patients were, on average, aged 66 ± 16 years, 50% were women, and 74% were white.
Figure 1.
Flow diagram of case selection. Abbreviation: ICU, intensive care unit.
Procalcitonin Practice Patterns
PCT was checked an average 1.6 ± 5.2 days after antibiotics were started. A histogram of PCT orders relative to antibiotic timing is shown in Supplementary Figure 1. Of patients who had a PCT checked, 1250 (33.2%) had a PCT repeated a mean 3.2 ± 4.6 days after the first PCT. Baseline clinical variables stratified by PCT use are shown in Table 1. In multivariable analysis among hospitals that used PCT, patient factors associated with PCT use were younger age, a longer length of stay, the presence of septic shock, metabolic acidosis or acute respiratory failure, a history of heart failure or peripheral vascular disease, and pneumonia as an infection source. No hospital-level factors were associated with PCT use. Surgical attending physicians were less likely to use PCT than medical, critical care, or cardiology physicians. PCT use varied widely among hospitals (use in 1%–95% of sepsis cases); the majority of variation in PCT use (intraclass correlation coefficient, 53%; 95% confidence interval [CI], 46%–61%) remained unexplained by measured patient or hospital characteristics.
Outcomes, Primary Analysis
In individual patient-level analysis, PCT orders were associated with more antibiotic DOT (multivariable-adjusted relative risk [aRR], 1.17; 95% CI, 1.15–1.18), increased rates of C. difficile infection (multivariable-adjusted odds ratio [aOR], 1.42; 95% CI, 1.09–1.85), and no difference in mortality (hazard ratio [HR], 1.05; 95% CI, 0.93–1.19). Antibiotic DOTs were associated with increased risk of C. difficile infection (no C. difficile, 7 days vs C. difficile, 12 days; P < .001 by Wilcoxon rank-sum test).
Sensitivity Analyses
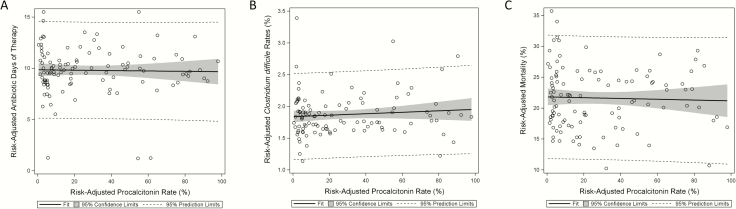
Sensitivity analyses showed similar patterns of results to the primary analysis. In the full cohort of hospitals, including hospitals that never measured PCT (71937 patients among 430 hospitals [Figure 1]), and in the subgroup of hospitals that had previously measured PCT in 2011, PCT remained associated with increased antibiotic DOT and C. difficile infection (Table 2). When length of stay was excluded from our models, PCT use remained associated with increased antibiotic DOT (aOR, 1.20; 95% CI, 1.19–1.22). Patients with serial PCT orders also showed higher rates of antibiotic use and C. difficile but no significant association with mortality (HR, 0.95; 95% CI, 0.83–1.12). In hospital-level PCT ecological exposure analyses among individual patients, admission to a hospital that checked PCT in at least 1 patient was not associated with a difference in mortality or antibiotic DOT but was associated with an increased incidence of C. difficile infection (aOR, 1.24; 95% CI, 1.06–1.45; Table 2). Increasing risk-adjusted rates of PCT use or serial PCT use among hospitals that used PCT were not associated with measured outcomes (Table 2). When stratified by site of infection, PCT orders remained associated with increased antibiotic use except in the case of patients with bacteremia, where there was no association (P for interaction < .0001; Supplementary Table 2). We similarly identified significant interactions between site of infection, PCT, and mortality but not C. difficile infection (Supplementary Table 2). Hospital risk-standardized rates of PCT testing did not show associations with hospital risk-standardized antibiotic days (r = −0.02; P = .76), C. difficile infection (r = 0.03; P = .75), or mortality (r = −0.04; P = .68; Figure 2). Difference-in-differences analysis demonstrated that patients admitted to hospitals that adopted PCT testing had similar changes in antibiotic days (aRR, 1.01; 95% CI, 0.99–1.02), C. difficile infection rates (aOR, 1.02; 95% CI, 0.96–1.08), and mortality (aOR, 1.00; 95% CI, 0.98–1.02) relative to patients in hospitals that did not adopt PCT (Table 2).
Table 2.
Patient Outcomes Associated With Procalcitonin Use
| Analysis | Antibiotic-Days | Clostridium difficile | Mortality |
|---|---|---|---|
| Primary analysis | |||
| Hospitals using PCT | 1.17 (1.15–1.18) | 1.42 (1.09–1.85) | HR 1.05 (0.93–1.19) |
| Sensitivity analyses | |||
| All hospitals | 1.16 (1.15–1.18) | 1.52 (1.19–1.95) | 0.92 (0.83–1.03) |
| Hospitals using PCT in 2011 and 2012 | 1.16 (1.15–1.18) | 1.51 (1.08–2.13) | 0.89 (0.77–1.03) |
| Hospitals using PCT, length of stay excluded from model | 1.20 (1.19–1.22) | ||
| Hospitals using PCT twice in at least 1 patient | 1.06 (1.04–1.07) | 1.43 (1.07–1.93) | 0.92 (0.81–1.05) |
| Patients with 2 or more PCT orders | 1.42 (1.39–1.45) | 1.74 (1.18–2.55) | HR 0.97 (0.83–1.14) |
| Difference-in-differences | 1.01 (0.99–1.02) | 1.02 (0.96–1.08) | 1.00 (0.98–1.02) |
| Comparison of hospitals using PCT vs not using PCT | 1.01 (0.93–1.08) | 1.24 (1.06–1.45) | 1.02 (0.92–1.14) |
| Increasing rates of hospital PCT use | 1.00 (0.99–1.00) | 1.00 (1.00–1.01) | 1.00 (1.00-1.00) |
| Increasing rates of hospital serial PCT use | 1.00 (0.99–1.01) | 1.01 (1.00–1.02) | 1.00 (0.99–1.00) |
Data presented as adjusted risk ratio (95% confidence interval [CI]) for antibiotic-days and adjusted odds ratio (95% CI) for Clostridium difficile and mortality, except where noted.
Abbreviation: HR, hazard ratio; PCT, procalcitonin.
Figure 2.
Associations between risk-adjusted hospital procalcitonin (PCT) rates and risk-adjusted hospital outcomes among hospitals that used PCT. A, Association between risk-adjusted PCT rates and risk-adjusted antibiotic days, r = −0.02, P = .76. B, Association between risk-adjusted PCT rates and risk-adjusted Clostridium difficile infection, r = 0.03, P = .75. C, Association between risk-adjusted PCT rates and risk-adjusted mortality, r = −0.04, P = .68.
DISCUSSION
Randomized, controlled trial [12] evidence supports the efficacy of PCT-guided antibiotic algorithms for decreasing antibiotic use, providing evidence for the potential utility of PCT as an aid to antibiotic stewardship programs. Current guidelines for antibiotic stewardship and treatment of sepsis include recommendations to use PCT to aid decisions regarding cessation of antibiotic therapy [14, 18]. We sought to characterize the translation of PCT trial results into real-world practice among critically ill patients with sepsis in the United States. Approximately 5% of patients in the United States with sepsis had PCT levels measured. However, when measured, PCT levels were checked sequentially in fewer than 1 in 3 patients on average, a practice that runs contrary to algorithms tested in randomized trials that investigated PCT levels to guide antibiotic duration during sepsis. Hospital variation in use of PCT during sepsis was wide and mostly unexplained by measured patient and hospital characteristics. Contrary to the results of randomized trials, PCT use was not associated with reduced antibiotic use, reduced rates of C. difficile infection, or improved mortality. Taken together, our findings suggest that PCT testing during sepsis has been poorly implemented into real-world practice, yielding few measurable benefits. Programs designed to improve implementation and close large gaps between clinical trial efficacy and real-world effectiveness of PCT during sepsis are warranted.
In clinical trials, PCT was used in conjunction with an algorithm to guide antibiotic duration. Clinical trial algorithms generally required sequential measurement of PCT to identify when levels reached thresholds that represent low risk for ongoing bacterial infection that signaled appropriate cessation of antibiotics [8–11]. Despite relatively modest adherence to the algorithm in the trials (up to 53% in the largest trial [10]), antibiotic duration was significantly decreased by 2–3 days among patients randomized to receive PCT-guided care [10].
Few prior studies have evaluated PCT use outside of the clinical trial setting. One retrospective study of critically ill surgical patients within 2 German hospitals showed that implementation of a PCT protocol was associated with a decrease in antibiotic duration [13]. Another study evaluated outcomes associated with PCT algorithm use in centers that previously participated in the ProHOSP trial, which evaluated use of PCT-based algorithms in lower respiratory tract infections outside of the ICU setting [30]. In former participants in the ProHOSP trial, compliance with PCT algorithms was poor among US centers (<40%) when compared to European centers (60%–80%); however, compliance with PCT algorithms was associated with decreased antibiotic duration (5.9 vs 7.4 days) without increased adverse events.
Several factors may contribute to the lack of benefits observed with PCT use in the United States as compared to European studies. Importantly, we observed that fewer than one third of patients who had PCT checked had serial levels drawn, demonstrating that real practice was inconsistent with the serial PCT measurements used successfully in the clinical trial setting. Our database does not provide a sufficient level of detail to know with certainty whether an individual hospital used a PCT-based algorithm. However, analyses of hospital rates of serial PCT orders (as a surrogate for potential use of PCT algorithms) did not find associations between increasing rates of serial PCT use and antibiotic use. Further, the high level of unexplained variation observed in our study suggests that PCT use may be idiosyncratic and clinician dependent, without coordination of practices between providers that may be required to decrease antibiotic use across care settings (eg, from transition between ICU and ward). Because our data source did not provide PCT levels, we could not determine whether treatment decisions were altered based on PCT results. Further study is necessary to determine how PCT levels influence treatment decisions.
Our study had several limitations to be considered in evaluating our findings. First, unmeasured confounding in observational research cannot be excluded. The association between PCT and increased antibiotic use and C. difficile rates in patient-level analyses, but not hospital-level analyses, suggests the possibility of unmeasured confounding by indication for PCT testing in patient-level analyses. Analyses that were better able to account for unmeasured confounding, such as the difference-in-differences analysis, showed no association between PCT use and these outcomes. Thus, while PCT may not necessarily be associated with worse outcomes, we were unable to identify associations between PCT use and real-world benefits. Further, association between antibiotic DOT and C. difficile infection provides potential mechanistic insights between PCT use and potentially increased C. difficile rates. Second, use of ICD9-CM coding may be subject to misclassification bias. For example, ICD-9CM codes for identifying C. difficile have been shown to have modest sensitivity and high specificity [26, 27]. A nondifferential misclassification of C. difficile diagnosis by ICD-9CM codes would result in an underestimation of the observed association between PCT and C. difficile infection, whereas the influence of potential differential misclassification on effect estimates is unclear. Third, our analysis was limited to patients in 2012, and the availability of rapid PCT results has likely increased since then. However, most trials that show efficacy of using PCT-based antibiotic use algorithms in the ICU were published prior to 2012. More centers have likely gained more experience in the use of PCT testing since 2012. Whether real-world outcomes associated with PCT use have changed since 2012 is unclear; subgroup analysis in centers with experience using PCT prior to 2012 also did not show benefit. Fourth, excluding patients from analyses due to missing data has the potential to bias effect estimates if reasons for missing data associate with outcomes. However, few patients in our dataset had missing data—1.1% were excluded from analyses due to missing data. Finally, we studied patients with sepsis present on admission to the hospital; our results may not apply to patients who developed nosocomial sepsis later during their hospitalization.
We used definitions of sepsis in use prior to Sepsis-3 [31] to identify our study cohort, which is in line with the sepsis definitions used in prior randomized trials of PCT. It is possible that some patients with suspected sepsis who may never have received antibiotics based on a PCT level were excluded from our cohort. However, methods to define a cohort of patients with suspected infection who had antibiotics withheld using claims data are unclear. Additionally, prior randomized trials did not find that PCT accurately identified critically ill patients with suspected infection who could have initiation of antibiotics safely withheld or that PCT decreased initiation of antibiotics in the ICU [10, 32]. Clinical practice guidelines recommend against use of PCT to guide decisions to start antibiotics among critically ill patients [18, 33]. As such, we evaluated PCT as a potential method to reduce duration of antibiotics among patients with suspected infection who received at least 1 dose of antibiotic.
In conclusion, PCT use among critically ill patients with sepsis in real-world settings was not associated with decreased antibiotic use or improved antibiotic-associated outcomes on average. Importantly, PCT was measured infrequently and most often only checked once, suggesting that PCT was not implemented according to protocols found efficacious in clinical trials. Our results suggest that studies to improve implementation of PCT are warranted prior to widespread adoption.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. The work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health (NIH; K01HL116768 to A. J. W.).
Potential conflicts of interest. A. J. W. reports grants from NIH and personal fees from UptoDate outside the submitted work. All other authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thorac Soc 2015; 12:216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312:90–2. [DOI] [PubMed] [Google Scholar]
- 3. Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14: 742–50. [DOI] [PubMed] [Google Scholar]
- 4. Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003; 163:972–8. [DOI] [PubMed] [Google Scholar]
- 5. Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 2004; 363:600–7. [DOI] [PubMed] [Google Scholar]
- 6. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med 2006; 174:84–93. [DOI] [PubMed] [Google Scholar]
- 7. Schuetz P, Christ-Crain M, Thomann R, et al. ; ProHOSP Study Group Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302:1059–66. [DOI] [PubMed] [Google Scholar]
- 8. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16:819–27. [DOI] [PubMed] [Google Scholar]
- 9. Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008; 177:498–505. [DOI] [PubMed] [Google Scholar]
- 10. Bouadma L, Luyt CE, Tubach F, et al. ; PRORATA trial group Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375:463–74. [DOI] [PubMed] [Google Scholar]
- 11. Hochreiter M, Köhler T, Schweiger AM, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care 2009; 13:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kopterides P, Siempos II, Tsangaris I, Tsantes A, Armaganidis A. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med 2010; 38:2229–41. [DOI] [PubMed] [Google Scholar]
- 13. Hohn A, Schroeder S, Gehrt A, et al. Procalcitonin-guided algorithm to reduce length of antibiotic therapy in patients with severe sepsis and septic shock. BMC Infect Dis 2013; 13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes of procalcitonin use in critically ill patients with sepsis. Abstract presented at 73rd Annual Meeting of the Massachusetts Pulmonary Section; 2016. March 3; Newton, MA. [Google Scholar]
- 16. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes of procalcitonin use in critically ill patients with sepsis. Abstract presented at American Thoracic Society 2016 International Conference; 2016. May 18; San Francisco, CA. [Google Scholar]
- 17. Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the Angus implementation of the International Consensus Conference definition of severe sepsis. Med Care 2014; 52: e39-e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005; 43:480–5. [DOI] [PubMed] [Google Scholar]
- 20. Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care 2004; 42:801–9. [DOI] [PubMed] [Google Scholar]
- 21. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 22. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–10. [DOI] [PubMed] [Google Scholar]
- 23. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348:1546–54. [DOI] [PubMed] [Google Scholar]
- 24. Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A; Co-operative Antimicrobial Therapy of Septic Shock Database Research Group Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med 2014; 189:1204–13. [DOI] [PubMed] [Google Scholar]
- 25. Kubin CJ, Jia H, Alba LR, Yoko Furuya E. Lack of significant variability among different methods for calculating antimicrobial days of therapy. Infect Control Hosp Epidemiol 2012; 33:421–3. [DOI] [PubMed] [Google Scholar]
- 26. Scheurer DB, Hicks LS, Cook EF, Schnipper JL. Accuracy of ICD-9 coding for Clostridium difficile infections: a retrospective cohort. Epidemiol Infect 2007; 135:1010–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dubberke ER, Reske KA, McDonald LC, Fraser VJ. ICD-9 codes and surveillance for Clostridium difficile-associated disease. Emerg Infect Dis 2006; 12:1576–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta AB, Cooke CR, Wiener RS, Walkey AJ. Hospital variation in early tracheostomy in the United States: a population-based study. Crit Care Med 2016; 44:1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol 2005; 162:1016–23. [DOI] [PubMed] [Google Scholar]
- 30. Albrich WC, Dusemund F, Bucher B, et al. ; ProREAL Study Team Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in “real life”: an international, multicenter poststudy survey (ProREAL). Arch Intern Med 2012; 172:715–22. [DOI] [PubMed] [Google Scholar]
- 31. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Layios N, Lambermont B, Canivet JL, et al. Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Crit Care Med 2012; 40:2304–9. [DOI] [PubMed] [Google Scholar]
- 33. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.