Fig. 9.
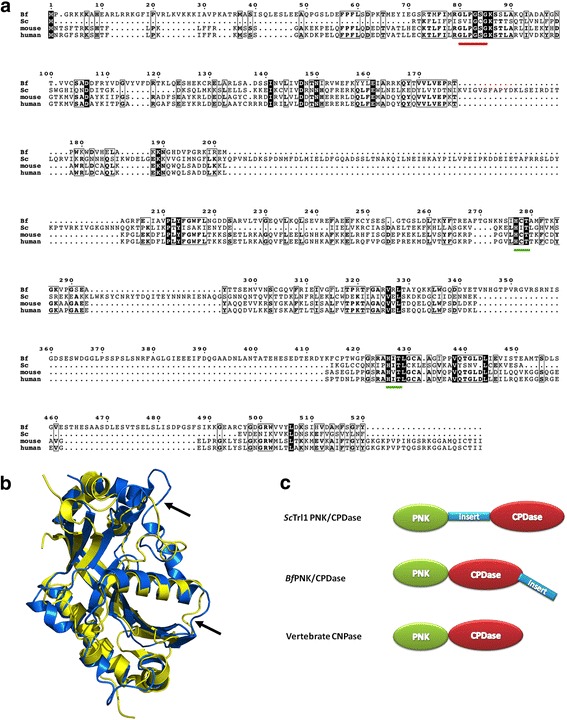
Domain arrangement of PNK/CPDase proteins. a Multiple sequence alignment using T-Coffee [54]. Aligned sequences of ScTrl1 PNK/CPDase (Sc), BfPNK/CPDase (Bf), MmCNPase (mouse), and HsCNPase (human). The N-terminal P-loop motif and two C-terminal H-x-(T/S)-x motifs are underlined in red and green, respectively. b Superposition of the homology model of the CPDase domain of BfPNK/CPDase (blue), generated using Phyre 2, and the crystal structure of the catalytic domain of MmCNPase (yellow) [PDB ID: 2YDB] [27, 53]. The black arrows point to the locations of flexible loops in the homology model of the CPDase domain of BfPNK/CPDase (blue). c The overall three-dimensional shapes of PNK/CPDase proteins determined in this study can be used to propose a structural arrangement of the domains
