Abstract
Cardiovascular dysfunction is highly comorbid with mood disorders, such as anxiety and depression. However, the mechanisms linking cardiovascular dysfunction with the core behavioral features of mood disorder remain poorly understood. In this study, we used mice bearing a knock-in sarcomeric mutation, which is exhibited in human hypertrophic cardiomyopathy (HCM), to investigate the influence of HCM over the development of anxiety and depression. We employed behavioral, MRI, and biochemical techniques in young (3–4 mo) and aged adult (7–8 mo) female mice to examine the effects of HCM on the development of anxiety- and depression-like behaviors. We focused on females because in both humans and rodents, they experience a 2-fold increase in mood disorder prevalence vs. males. Our results showed that young and aged HCM mice displayed echocardiographic characteristics of the heart disease condition, yet only aged HCM females displayed anxiety- and depression-like behaviors. Electrocardiographic parameters of sympathetic nervous system activation were increased in aged HCM females vs. controls and correlated with mood disorder–related symptoms. In addition, when compared with controls, aged HCM females exhibited adrenal gland hypertrophy, reduced volume in mood-related brain regions, and reduced hippocampal signaling proteins, such as brain-derived neurotrophic factor and its downstream targets vs. controls. In conclusion, prolonged systemic HCM stress can lead to development of mood disorders, possibly through inducing structural and functional brain changes, and thus, mood disorders in patients with heart disease should not be considered solely a psychologic or situational condition.—Dossat, A. M., Sanchez-Gonzalez, M. A., Koutnik, A. P., Leitner, S., Ruiz, E. L., Griffin, B., Rosenberg, J. T., Grant, S. C., Fincham, F. D., Pinto, J. R. Kabbaj, M. Pathogenesis of depression- and anxiety-like behavior in an animal model of hypertrophic cardiomyopathy.
Keywords: adrenal gland, heart rate variability, mood disorder, sarcomeric protein, troponin
Hypertrophic cardiomyopathy (HCM) is a clinical syndrome characterized by left ventricle wall hypertrophy, reduced ventricle volumetric capacity, and diastolic dysfunction (1). HCM is present in as many as 1:200 adults and is an autosomal-dominant inherited trait that affects genes coding for proteins of the cardiac sarcomere and is the leading cause of sudden cardiac death in young people (1–4). HCM has been documented in all ages and in both sexes (5), and in most cases, it is the result of a mutation of the sarcomeric protein (6). Sarcomeric mutations in cardiac troponin T, I, and C (cTnT, cTnI, and cTnC, respectively) have been documented in humans with an HCM phenotype (7, 8). Recently, we generated a knock-in mouse bearing an HCM mutation in TNNC1 (gene encoding cTnC), which is expressed in the heart and slow skeletal muscles (9). Since its identification <10 yr ago (10), there have been additional reports of humans with the Ala8Val mutation in the TNNC1 gene, as well as phenotypic symptoms associated with HCM and restricted cardiomyopathy (RCM) (9, 11, 12). As has been shown for other HCM troponin-related mutations, the cTnC-Ala8Val mutation in the murine heart induces cardiac interstitial fibrosis, myofibrillar disarray, left atrial enlargement, diastolic dysfunction, and an increased ejection fraction (EF) (9, 13–16). Thus, this mutation and its associated functional alterations significantly contribute to the development of HCM/RCM and its associated symptoms, as is observed in the human population.
There are many reports that individuals with HCM are at a higher risk for anxiety and depression, compared with the general population (17–20). This difference has also been demonstrated in individuals with heart failure and other forms of cardiovascular disease (21, 22). The comorbid expression of a heart condition and mood disorder significantly worsen quality of life (23). Furthermore, depression has been shown to exacerbate physical illness (24), and the severity of depression can significantly predict cardiac events and mortality in patients with heart disease (25–28). In addition to what has been shown clinically, there is substantial preclinical evidence linking a depressive-like phenotype and cardiovascular dysfunction in rodents. Coronary artery ligation reliably induces a depressive-like phenotype in rodents (29–33), which can be reversed by anti-inflammatory drugs and antidepressant drugs (32, 34). Although these methods can replicate a behavioral phenotype associated with depression and have identified some neural changes associated with inflammation and apoptosis (31, 34), the short duration of cardiovascular stress makes them relatively poor models of human cardiovascular disease. Both acute and chronic social and physical stress can induce expression of cardiovascular dysfunction (35), and do so in parallel with the induction of mood disorder–related symptoms (36–38). These, and other studies, support the bidirectional relationship between cardiovascular dysfunction and mood disorder.
The preponderance of individuals who suffer from depression are female (39), which has also been reported in the population with heart disease (22, 40, 41). However, how genetic sex influences cardiovascular pathophysiology and response to therapeutics is still not fully understood (42). Accordingly, one aim of the present study was to use female mice to determine whether a previously established knock-in mouse model of HCM, bearing a sarcomeric mutation found in HCM patients (9–11), displays core behavioral features of mood disorder. We also investigated, in the same animals, sympathetic nervous system (SNS) activity, adrenal gland weight, plasma corticosterone (Cort), and IL-6 levels since these factors have been shown to be dysregulated in individuals who have cardiovascular insults and psychological stress (43–46).
The precise brain regions that influence mood and are sensitive to the effects of HCM remain to be determined, yet there are some promising candidate regions. The prefrontal cortex (PFC) plays a key role in emotion regulation (47), and many individuals with depression exhibit reduced activity and decreased volume of this brain region (48, 49). The caudate putamen (CPu) has also been shown to exhibit depression-related volumetric reductions (50) and compromised tissue integrity in those with heart failure comorbid with depression (51). Another brain region that is negatively affected by stress and exhibits reduced volume in patients with major depression is the hippocampus (HPC) (45, 52). Therefore, another aim of this study was to use MRI to determine whether structural changes in mood-related brain regions were present in mice with HCM vs. controls.
It is well known that HPC homeostasis and plasticity are regulated via activity-dependent release of BDNF, which targets downstream second-messenger signaling cascades, such as MAPK and Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα). The MAPK and CaMKIIα intracellular signaling cascades promote cellular excitation and synthesis of synaptic proteins that are critical for normal plasticity and overall neurotrophic health (53–56). Reductions in these neuroplasticity-related proteins have been reported in the HPC of patients who have major depression (52). As such, in this study, we examined levels of these neuroplasticity markers within the HPC to determine whether this brain region exhibits functional changes in mice with HCM.
MATERIALS AND METHODS
Animals
Knock-in homozygous mice bearing the mutation Ala8Val in cTnC, KI-TnC-A8V+/+ (HCM), and wild-type (control) mice were generated as has been described (9). In brief, embryonic stem cells were electroporated with 25 μg of linearized DNA harboring the A8V mutation. Embryonic stem cell clones that underwent successful homologous recombinantion of the A8V mutation in cTnC were injected into C57BL/6 blastocysts. Male progeny with a high percentage of coat color chimerism were bred to C57BL/6 females to establish germ line transmission. Heterozygous mice (KI-TnC-A8V+/−) were mated with MHC-Cre transgenic mice to delete the neomarker. The homozygous KI-TnC-A8V+/+ mice were obtained by intercrossing the A8V heterozygous mice [for further details on KI mouse generation and characterization, see Martins et al. (9)]. Adult females were pair housed in a temperature-controlled vivarium on a 12:12 h light–dark cycle in microinsulated boxes. The animals had ad libitum access to distilled water and Purina 5001 pellet chow (Purina; St. Louis, MO, USA). Two age groups were examined: young adult (age, 3.9 ± 0.6 mo, n = 8/group) and aged adults (7.2 ± 1.8 mo, n = 13–16/group). Because heterozygous males do not exhibit evidence of HCM until 14 mo of age (9), we used homozygous mice to investigate the impact of HCM in young adult and aged adult females, in the absence of other aging-related health declines. Another group of aged adult control and HCM females were used for MRI volumetric analyses (age, 8.4 ± 0.2 mo, n = 4/group). Mice were handled every other day to habituate them to handling before behavioral testing. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, D.C., USA).
Echocardiography measurements
Echocardiography (echo) was performed with a Vevo 2100 high-resolution in vivo imaging system (FujiFilm Sonosite, Inc., Toronto, ON, Canada) (57). Young adult and aged adult mice were anesthetized with 2% isoflurane in 1 L oxygen/min inhaled continuously during the procedure. M-mode imaging of the parasternal short-axis view determined systolic and diastolic left ventricle dimensions, as well as anterior and posterior wall thickness. M-mode imaging was used to determine fractional shortening and EF. An apical 4-chamber view was used to acquire mitral valve (MV) flow parameters. Pulsed-wave spectral Doppler was used to measure MV inflow, as well as its 2 main waves, early and late atrial, from which we calculated deceleration time, isovolumetric (IV) relaxation (IVRT), and IV contraction time (IVCT). MV inflow parameters were used as an index of diastolic function (58).
Electrocardiography measurements
Electrocardiographic (ECG) measures were collected from conscious aged adult females, with the ECGenie System Recording Platform (Mouse Specifics, Inc., Framingham, MA, USA) (57). An array of gel-coated ECG electrodes (Red Dot Diagnostic ECG Electrodes; 3M, St. Paul, MN, USA) was embedded in the platform floor and positioned 3 cm apart to provide contact between the electrodes and the animals’ paws. Mice were gently removed from cages, placed on the ECG recording platform, and given 30 min to acclimate [heart rate (HR) equilibrium period]. After this equilibrium period, ECG signals were recorded for 2–3 s (25–39 QRS complexes), while the mouse passively established contact with its paws and the electrodes. Data acquisition was performed with the Mouse Acquisition Module (Mouse Specifics, Inc.). These signals were digitized with a 16-bit presampling rate of 2500 Hz. Analysis of individual ECGs was performed with the EzCG Analysis Software Package (Mouse Specifics, Inc.). Body temperatures were measured with an infrared thermometer to verify consistency before and after ECG readings. We examined the cardiac parasympathetic (vagal) modulation of HR variance (HRV) according to the established guidelines (59), through the time domains total power (TP), percentage of adjacent R-wave to R-wave (R–R) intervals that differ by a length of time exceeding the experimental threshold of 6 ms (similar to pNN50 in humans), and root-mean square of the successive differences (RMSSDs) R–R intervals. These are considered markers of cardiac vagal modulation. The frequency domains, high-frequency [HF (1.5–4.0 Hz), vagal modulation] and low-frequency [LF (0.4–1.5 Hz), vagal-sympathetic interplay and baroreflex function] components (60) of the HRV parameters were calculated by means of fast Fourier transformation.
Behavioral analysis
Light–dark box test
The light–dark box is composed of 2 compartments (dark: 200 × 310 mm; white: 310 × 310 mm) connected by an 8-cm-wide × 14-cm-high opening located at floor level (Model LE-812; Bioseb, Pinellas Park, FL, USA). The floor and walls of one compartment were black and illuminated by a red light (4.5 lux), the floor and walls of the other compartment were white and illuminated by a light bulb at the top of the box (10 lux). Mice were transferred to a dimly lit test room (4.5 lux) and allowed to acclimate to the environment for 30 min prior to behavioral testing. The test was initiated by placing a mouse in the dark compartment, and the animal was given 5 min to freely explore both compartments. Mice were tested individually and the box was cleaned with 70% EtOH between subjects to remove any odorant cues. All sessions were video recorded by a camera above the light–dark box and saved for analysis. A trained observer who was blind to genotype scored the time spent in the light side and the number of transitions between the compartments. Any animal that failed to enter the white compartment during the test was excluded from analysis.
Forced swim test
Mice were transferred to the dimly lit test room and given 30 min to acclimate before the start of the forced swim test (FST). The mice were placed in a 4 L Pyrex glass beaker filled with 3 L of water at 24 ± 1°C (61) for 6 min. FST sessions were recorded and by video camera and saved; the beakers were cleaned with 70% EtOH, and water was changed between subjects. A trained observer who was blinded to genotype quantified the time spent immobile during the final 4 min of the FST.
Terminal procedures
The body weight of each mouse was recorded at the onset of the light cycle, and within the first 2 h of the light cycle, mice were euthanized via rapid decapitation. Trunk blood was collected into tubes containing chilled 0.5 M EDTA (Sigma-Aldrich, Milwaukee, WI, USA) and stored on ice, and plasma was extracted via refrigerated centrifugation (2800 rpm for 20 min) chilled to 4°C. Plasma was transferred to sterile, prechilled microcentrifuge tubes and stored at −80°C for processing. Adrenal glands were bilaterally excised, placed in a sterile preweighed microcentrifuge tube, and placed directly onto dry ice; adrenal gland weight was calculated with the formula: [adrenal gland weight (mg)/body weight (g) × 1000]. The brains were rapidly removed and frozen in 2-methyl butane (Sigma-Aldrich) on dry ice at −20°C and stored at −80°C for processing.
Cort radioimmunoassay
Plasma Cort concentrations were measured using the radioimmunoassay (RIA) Corticosterone Coat-A-Count Kit according to the manufacturer’s instruction (TKRC1; Siemens, Dublin, Ireland). The minimum detectable level of Cort with this kit is 7.7 ng/ml; samples were run in duplicate, and any samples with a coefficient of variation (CV) >10% were excluded from analysis.
IL-6 ELISA
Plasma IL-6 concentrations were measured using the Legend Max Mouse IL-6 ELISA Kit according to the manufacturer’s instruction (431307; BioLegend, San Diego, CA, USA). The minimum detection limit of the kit is 2 pg/ml; samples were run in duplicate and any samples with a CV >10% were excluded from analysis.
Ex vivo MRI analysis of brain region volumes in aged control and HCM females
Females underwent vaginal lavage every day for at least 2 estrous cycles to confirm normal cycling and determine the estrous stage. When in diestrus 1, mice were anesthetized (90 mg/kg, i.p. ketamine and 5 mg/kg xylazine) and transcardially perfused with 0.2 M phosphate-buffered saline (PBS) (sodium chloride, S7653; Sigma-Aldrich) followed by 4% paraformaldehyde (PFA) (19210; Electron Microscopy Sciences, Hatfield, PA, USA), brains were then carefully removed from the skull. The brains were submerged in 4% PFA for at least 48 h, and 24 h before the MRI, the brains were washed in 1× PBS to rinse out the PFA. In a 10 mm NMR tube, the brain was submerged in Fluorinert (3M), a nonproton compound with no MRI signal. All MRI were acquired with an 11.75-T magnet with an 89-cm bore located at the Florida A&M University–Florida State University College of Engineering (Tallahassee, FL, USA). The system is equipped with a Bruker Avance Console and Paravison 3.0 (Bruker Corp., Billerica, MA, USA). A 10-mm birdcage coil tuned to 500 MHz was used for all imaging. A gradient-recalled echo sequence was used to analyze the brain anatomy. The scans were acquired with a 50-µm isotropic resolution acquired in sagittal orientation. Repetition time and echo time were set to 15 and 350 ms, respectively. A 50-µs block pulse with a 500-Hz bandwidth was used, and effective spectral width was set to 20 kHz. All data were analyzed in Amira (FEI Software, Hillsboro, OR, USA). Brains were registered to a common selected reference brain, to allow for accurate analysis and comparison. Using a brain atlas (62) the PFC, CPu, and HPC were segmented. The PFC was segmented from +2.77 to +1.41 mm from the bregma, the CPu was segmented from +1.69 to −1.91 mm from the bregma, and the HPC was segmented from −0.95 to −3.79 mm from the bregma. Volumetric data were recorded and mean volumetric data calculated. Brain region volumes were normalized using the formula: [volume of region of interest/(whole brain volume/body weight)].
Western blot analysis
Coronal cryostat sections containing the HPC were collected at 200 µm, and 1-mm tissue punches were collected from the dorsal HPC. Protein was extracted using the Tri Reagent protocol (Molecular Research Center, Cincinnati, OH, USA), and concentration was determined via Bradford Assay (500-0001; Bio-Rad, Philadelphia, PA, USA). Twenty micrograms of protein per sample was resolved by 12% acrylamide gel and subsequently transferred to a nitrocellulose membrane at 70 V for 2 h (Amersham Protran BA83; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Membranes were incubated with 5% milk in TBS for 1 h at room temperature and incubated overnight at 4°C with the following primary antibodies (all from Cell Signaling Technology, Danvers, MA, USA, unless otherwise specified): rabbit anti-GAPDH (1:2000, 5174; Cell Signaling Technology), rabbit anti-BDNF (1:500, sc-546; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit anti-p-MAPK (1:2000, 9101S), rabbit anti-MAPK (1:2000, 9102S), rabbit anti-p-CaMKIIα (1:2000, 12716), and mouse anti-CaMKIIα (1:2000, 50049). Membranes were incubated with donkey anti-rabbit or goat anti-mouse secondary antibody diluted 1:10,000 (926-68023 and 926-32210, respectively; Li-Cor Biosciences, Lincoln, NE, USA) for 45 min at room temperature. After all incubation steps, membranes were rinsed once with TBS-Tween (TBST) and 4 times with TBS on a rocking platform at room temperature. Membranes were imaged with an Odyssey infrared imaging system (Li-Cor Biosciences), and quantification was performed with ImageJ software (National Institutes of Health, Bethesda, MD, USA). The amount of protein for BDNF, MAPK, and CaMKIIα was calculated as the ratio of the optical density (OD) of the target band over the OD of the GAPDH band. Phosphorylated levels of MAPK and CaMKIIα were calculated as a ratio of the OD of the phosphorylated band over the OD of the total band. OD values were normalized to control values within each respective age group.
Statistical analysis
Data are reported as means ± sem. Student t tests were used for analysis of effect of genotype to influence variables of interest. Correlations were performed to examine the relationship between behavioral indices of mood disorder with RMSSD and with biochemical characteristics of the HPC. Any value >2 sd above the group mean was regarded as an outlier and was removed from the analysis. A value of P < 0.05 indicated significant results.
RESULTS
Echo confirmation of HCM
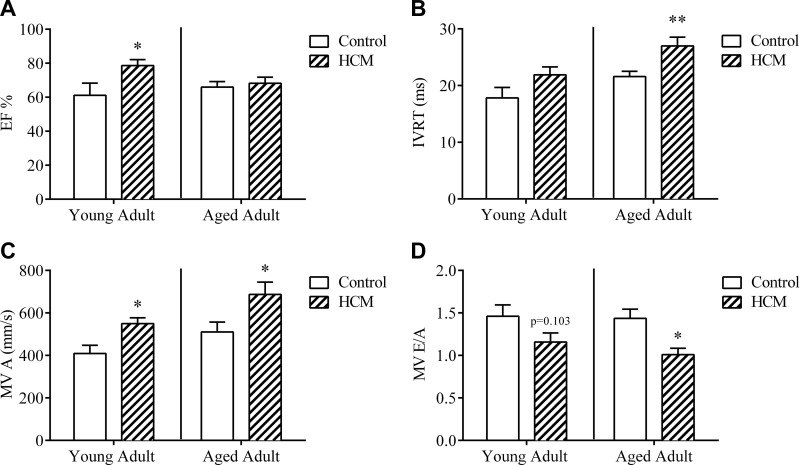
As summarized in Table 1 and Fig. 1, echo confirmed structural and hemodynamic changes induced by the HCM mutation in young adult females. There were no significant differences between young control and HCM mice for HR [t (11) = 1.277; P > 0.05], aortic ejection time (AET) [t (10) = 1.057; P > 0.05], IVCT [t (10) = 0.529; P > 0.05], nor was there an effect of genotype over MV early peak flow velocity (MV E) [t (10) = 0.602; P > 0.05]. HCM mice displayed trends toward higher IVRT ([t (10) = 1.77; P = 0.1071], higher fractional shortening (FS) [t (11) = 2.167; P = 0.053], lower MV early peak flow velocity/late atrial peak flow velocity MV E/A) [t (10) = 1.795; P = 0.1029], and a trend toward higher ventricular volume during diastole [t (11) = 2.14; P = 0.0556)] as compared with controls. Finally, young adult HCM mice displayed significantly higher EF [t (11) = 2.32; P < 0.05], higher MV late atrial peak flow velocity (MV A) [t (10) = 2.984; P < 0.05], and lower left ventricular volume during systole [t (11) = 2.459; P < 0.05], as compared to age-matched controls, which is indicative of diastolic dysfunction in young HCM mice.
TABLE 1.
Summary of echo data from young adult control and HCM mice
| Variable | Control | HCM | P |
|---|---|---|---|
| AET (ms) | 54.12 ± 2.616 | 57.824 ± 2.332 | 0.3155 |
| EF (%) | 61.086 ± 7.238 | 78.771 ± 3.268 | 0.0406* |
| FS (%) | 33.54 ± 5.23 | 47.202 ± 3.511 | 0.053 |
| HR (bpm) | 426.6 ± 21.11 | 394.04 ± 14.26 | 0.228 |
| IVCT (ms) | 20.648 ± 2.004 | 18.519 ± 3.492 | 0.6084 |
| IVRT (ms) | 17.824 ± 1.835 | 21.898 ± 1.39 | 0.1071 |
| MV A (mm/s) | 408.971 ± 35.6 | 549.85 ± 25.3 | 0.017* |
| MV E (mm/s) | 584.84 ± 53.9 | 646.383 ± 77.8 | 0.36 |
| MV E/A | 1.462 ± 0.132 | 1.157 ± 0.107 | 0.1029 |
| Volume diastole (µl) | 65.914 ± 8.7 | 43.697 ± 5.021 | 0.0556 |
| Volume systole (µl) | 25.066 ± 6.649 | 9.078 ± 1.916 | 0.0317* |
Data are means ± sem (n = 6 control; n = 6–7 HCM). *P < 0.05 vs. control.
Figure 1.
Echo variables in HCM young adult and aged females. EF percentage (A), IVRT (B), MV A (C), and MV E/A (D) in young adult and aged adult control and HCM females. Data are means ± sem; n = 6–7/group young adult, n = 9–13/group aged adult. *P < 0.05, **P = 0.005 vs. control within same age group.
Table 2 contains echo variables obtained from aged adult female mice (Fig. 1). Student’s t test revealed significantly lower HR in HCM as compared to control mice (t (20) = 2.212; P < 0.05), indicating bradycardia in HCM mice under anesthesia. Aged HCM mice displayed altered cardiac contraction and relaxation, demonstrated by higher IVRT [t (20) = 3.163; P < 0.005] and lower IVCT [t (20) = 5.278; P < 0.001] vs. controls. HCM aged mice displayed higher MV A [t (20) = 2.183; P < 0.05] and lower MV E/A [t (20) = 2.769; P < 0.05] vs. controls; alterations in these parameters indicate diastolic dysfunction in HCM animals. Aged HCM mice exhibited significantly poorer left ventricle myocardial performance IV (LV MPI) [t (20) = 3.355; P < 0.005] and increased LV MPI nonfilling time vs. controls [t (20) = 3.082; P < 0.01]. In addition, aged HCM females trended toward significantly longer AET [t (20) = 1.684; P = 0.1077] as compared to controls. There was no significant effect of genotype on EF [t (20) = 0.329; P > 0.05], FS [t (20) = 0.359; P > 0.05], or MV E [t (20) = 0.036; P > 0.05].
TABLE 2.
Summary of echo data from aged adult control and HCM mice
| Variable | Control | HCM | P |
|---|---|---|---|
| AET (ms) | 49.57 ± 1.97 | 54.48 ± 2.04 | 0.1077 |
| EF (%) | 65.92 ± 3.46 | 67.66 ± 4.41 | 0.7459 |
| FS (%) | 36.40 ± 2.54 | 37.81 ± 3.38 | 0.7235 |
| HR (bpm) | 449.67 ± 9.71 | 419.82 ± 9.50 | 0.0387* |
| IVCT (ms) | 27.00 ± 1.93 | 13.43 ± 1.31 | <0.0001* |
| IVRT (ms) | 21.58 ± 0.98 | 27.38 ± 1.74 | 0.005* |
| LV MPI IV | 1.00 ± 0.05 | 0.76 ± 0.04 | 0.0032* |
| LV MPI NFT | 1.02 ± 0.05 | 0.82 ± 0.03 | 0.0059* |
| MV A (mm/s) | 510.47 ± 48.18 | 676.53 ± 66.32 | 0.0412* |
| MV E (mm/s) | 687.38 ± 40.70 | 684.72 ± 72.60 | 0.9715 |
| MV E/A | 1.44 ± 0.11 | 1.03 ± 0.09 | 0.0118* |
Data are means ± sem (n = 13 control; n = 9 HCM). *P < 0.05 vs. control.
ECG confirmation of HCM-related SNS dysfunction
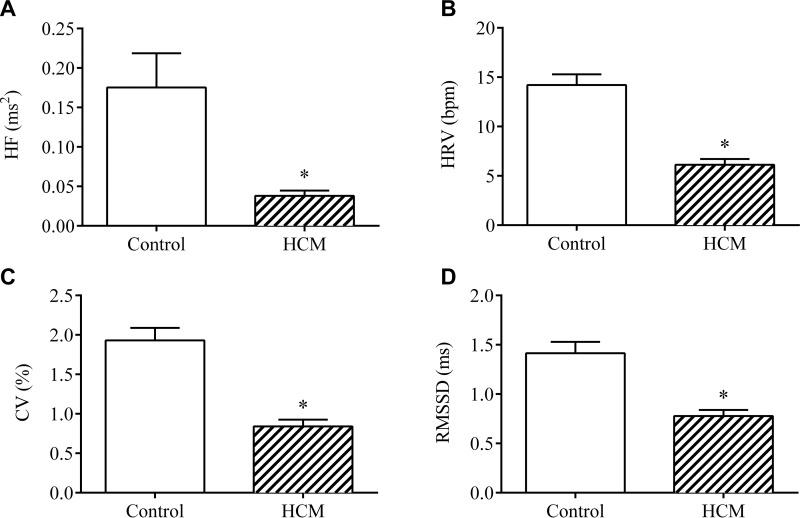
As summarized in Table 3 and Fig. 2, we used ECG to examine the electrical activity of the heart of aged adult mice. Student’s t test comparisons in aged mice revealed no significant difference between genotypes with regard to HR [t (21) = 0.737; P > 0.05] or in R–R intervals [t (21) = 0.618; P > 0.05]. In contrast, aged HCM mice exhibited significantly lower HRV [t (21) = 5.984; P < 0.05], lower coefficient of variation (CV) [t (21) = 5.555; P < 0.0001], and lower RMSSD [t (21) = 4.483; P < 0.0005] vs. controls, which indicates compromised parasympathetic tone in the HCM females. Similarly, aged HCM mice exhibited lower levels of total power [t (21) = 3.315; P < 0.005], reduced LF component of HRV [t (21) = 3.103; P < 0.01], and reduced HF component of HRV [t (21) = 2.751; P < 0.05] as compared to aged-matched controls. Taken together, these results serve as evidence of SNS excitation and reduced efferent vagal activity in aged adult mice with HCM.
TABLE 3.
Summary of electrocardiographic data from aged adult control and HCM mice
| Variable | Control | HCM | P |
|---|---|---|---|
| CV (%) | 1.93 ± 0.16 | 0.84 ± 0.08 | <0.0001* |
| HF (ms2) | 0.18 ± 0.04 | 0.04 ± 0.01 | 0.017* |
| HR (bpm) | 744.15 ± 11.51 | 733.64 ± 7.55 | 0.26 |
| HRV (bpm) | 14.21 ± 1.13 | 6.13 ± 0.59 | <0.001* |
| LF (ms2) | 0.43 ± 0.11 | 0.06 ± 0.02 | 0.003* |
| RMSSD (ms) | 1.41 ± 0.12 | 0.78 ± 0.06 | 0.0007* |
| R-R (ms) | 80.91 ± 1.27 | 81.89 ± 0.08 | 0.27 |
| TP (ms2) | 0.71 ± 0.15 | 0.14 ± 0.03 | 0.002* |
Data are means ± sem (n = 13 control; n = 10 HCM). *P < 0.05 vs. control.
Figure 2.
Electrocardiographic variables in HCM aged females. The HF component of HRV (A), HRV (B), CV (C), and RMSSD (D) between heartbeats in young adult and aged adult control and HCM female mice. Data are means ± sem (n = 10–13/group). *P < 0.05 vs. control.
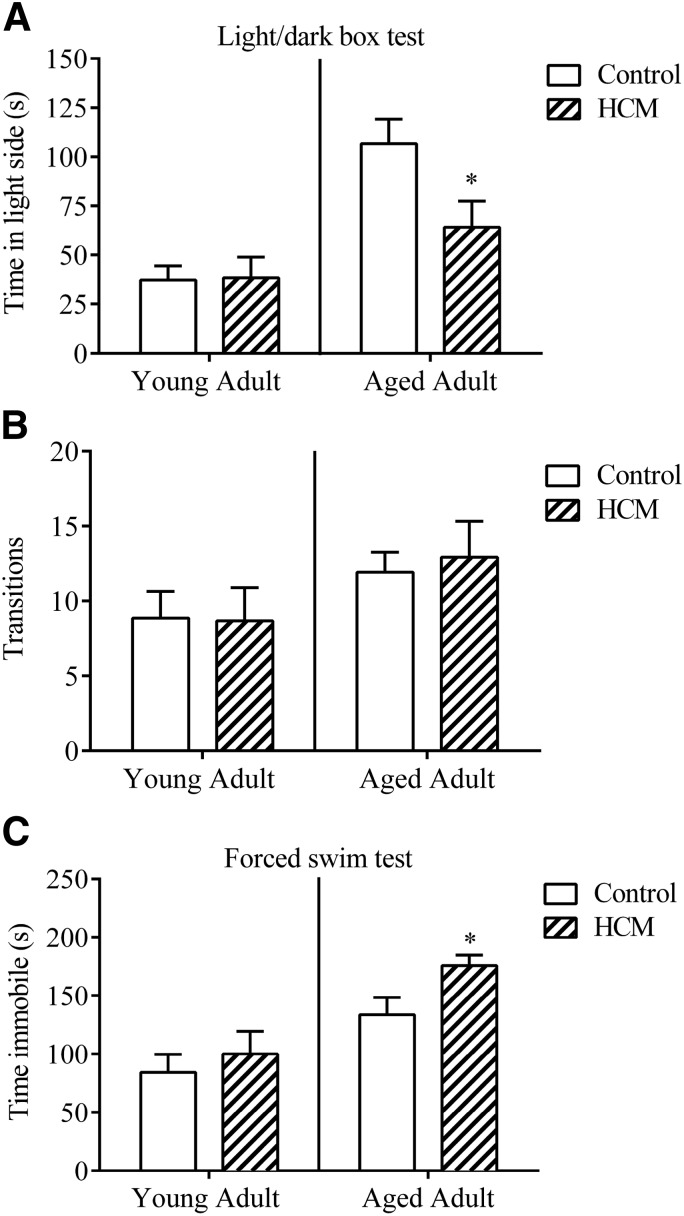
Light–dark box test revealed HCM-induced anxiety-like behavior in aged females
We observed no effect of genotype in young mice to influence time spent in the light chamber [t (11) = 0.093; P > 0.05], or transitions between the light and dark compartments [t (11) = 0.067; P > 0.05; Fig. 3A]. In contrast, aged controls spent more time in the light side as compared to aged HCM mice [t (24) = 2.351; P < 0.05], yet they did not differ in the number of transitions made between the 2 compartments [t (24) = 0.373; P > 0.05; Fig. 3B].
Figure 3.
Light–dark box test and FST reveal HCM-related mood disorder-like phenotype in aged female mice. A) Anxiety-like behavior in the light–dark box test. B) Number of transitions between the light and dark compartments. C) Depression-like behavior in the FST. Data are means ± sem; light–dark box test (n = 6–7/group, young adult; n = 12–14/group, aged adult; FST n = 8/group, young adult; n = 13–16/group, aged adult). *P < 0.05 vs. control.
FST revealed HCM-induced depression-like behaviors in aged females
Young adults exhibited no significant effect of genotype to influence immobility time in the FST [t (14) = 0.636; P > 0.05; Fig. 3C]. In contrast, aged adults with HCM exhibited significantly higher durations of immobility as compared to controls [t (27) = 2.305; P < 0.05].
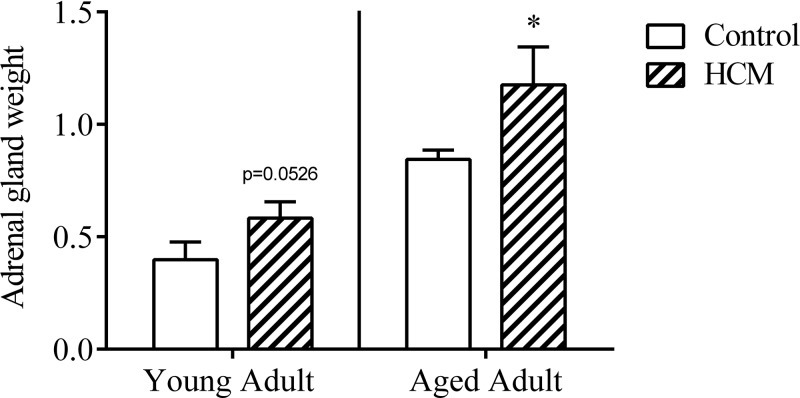
HCM-induced adrenal gland hypertrophy in females with HCM
There was a trend toward a significant effect of genotype to influence adrenal gland weight in young mice [t (14) = 1.732; P = 0.0526; Fig. 4]. Within the aged adults, controls exhibited significantly smaller adrenal glands as compared to aged HCM counterparts [t (22) = 2.212; P < 0.05].
Figure 4.
HCM induced adrenal gland hypertrophy in aged female mice: (adrenal gland weight/body weight) × 1000. Data are means ± sem (adrenal gland: n = 8/group, young; n = 10–14/group, aged). *P = 0.05 vs. control.
HCM did not influence levels of plasma Cort
Neither young adult [t (14) = 0.5834; P > 0.05; Supplemental Fig. 1A] nor aged adults [t (23) = 0.1106; P > 0.05] exhibited a significant effect of genotype to influence plasma Cort levels.
HCM did not affect levels of plasma IL-6
We observed no significant effect of genotype to influence levels of plasma IL-6 in young adults [t (12) = 0.6046; P > 0.05] or in aged adults [t (19) = 0.3984; P > 0.05; Supplemental Fig. 1B].
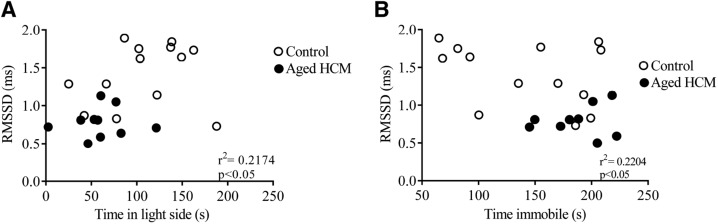
Altered autonomic nervous system activity correlated significantly with anxiety- and depression-like behaviors in aged adult mice
To examine the relationship between physiologic and behavioral variables, we ran a correlation analysis for RMSSD with duration in the light side of the light–dark box and with time spent immobile in the FST. We focused our attention on RMSSD, because this variable is a measure of the parasympathetic influence over the heart (63, 64). We examined the relationship between these variables in the aged adult mice, because this age group displayed an HCM-related anxiety- and depression-like phenotype. Significant relationships were observed for RMSSD and anxiety-like behavior in the light–dark box test (r2 = 0.2174; P < 0.05; Fig. 5A) and between RMSSD and depression-like behavior in the FST (r2 = 0.2204; P < 0.05; Fig. 5B).
Figure 5.
Autonomic nervous system activity correlates significantly with behavioral indices of mood disorder in aged adult females. A) RMSSD and its correlation with anxiety-like behavior in the light–dark box test. B) RMSSD and its correlation with depression-like behavior in the FST. Data are means ± sem (n = 23 for light–dark box test; n = 22 for FST).
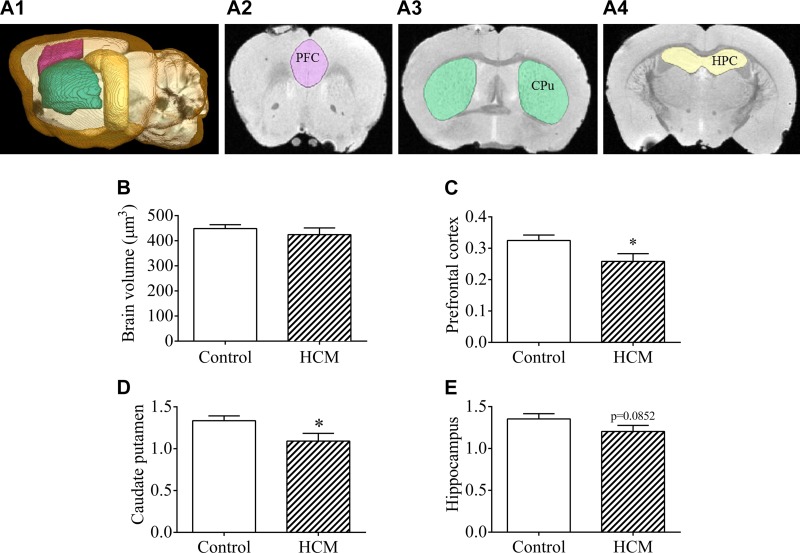
Ex vivo MRI analysis of HPC volume in aged control and HCM females
Figure 6A provides an outline of the areas of interest examined via MRI. There were no significant differences between control and HCM aged females with regard to whole brain volume in cubic millimeters [t(6) = 0.7877; P > 0.05; Fig. 6B]. HCM mice exhibited a significantly smaller PFC index [t(6) = 2.182; P < 0.05; Fig. 6C] and CPu index [t(6) = 2.221; P < 0.05; Fig. 6D] and a trend toward a smaller HPC, as compared to controls [t(6) = 1.557; P = 0.0852; Fig. 6E].
Figure 6.
Volumetric analysis of mood-related brain regions in aged adult HCM females. A1–A4) Sagittal section with 3-dimensional rendering of analyzed brain regions (A1), representative coronal sections depicting the PFC (A2), the CPu (A3), and the HPC (A4). B) Total brain volume in aged adult control and HCM mice. C–E) Indices of the PFC (C), CPu (D), and HPC of aged adult control and HCM females (E). Data are means ± sem (n = 4/group). *P < 0.05 vs. control.
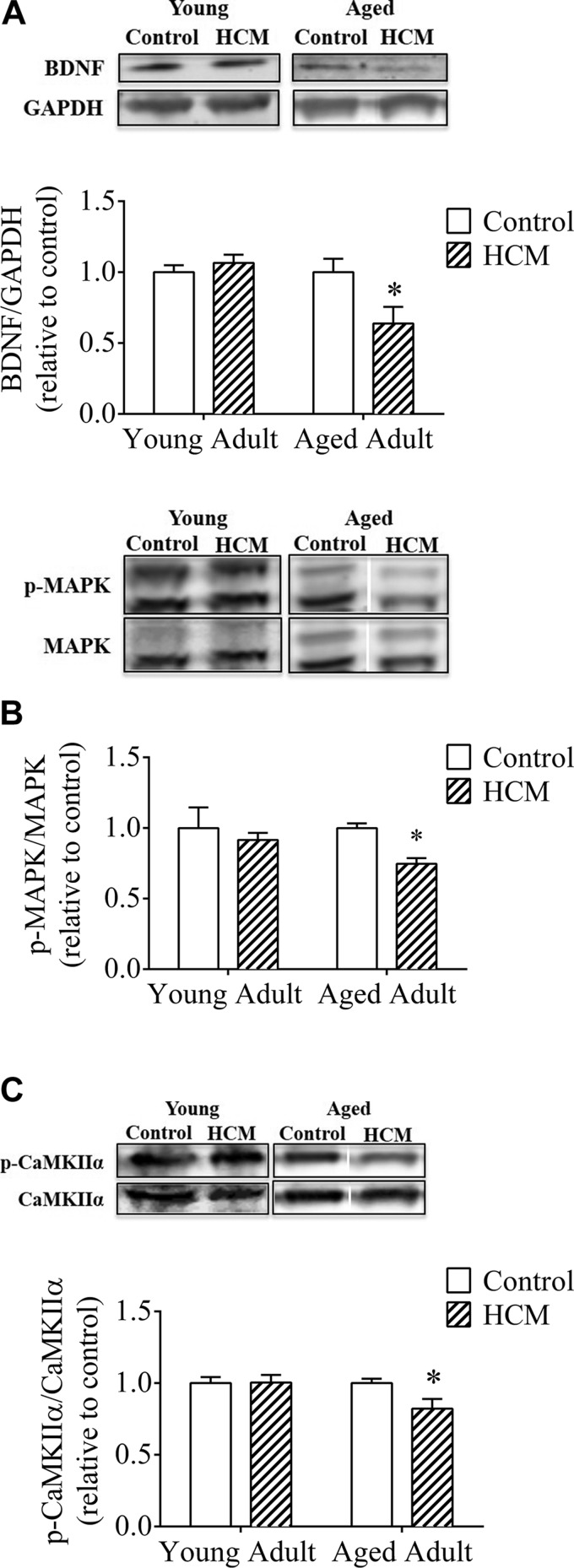
Markers of neurotrophic health and neuroplasticity were downregulated in the HPC of aged adults with HCM, yet were unaffected by HCM in young adults
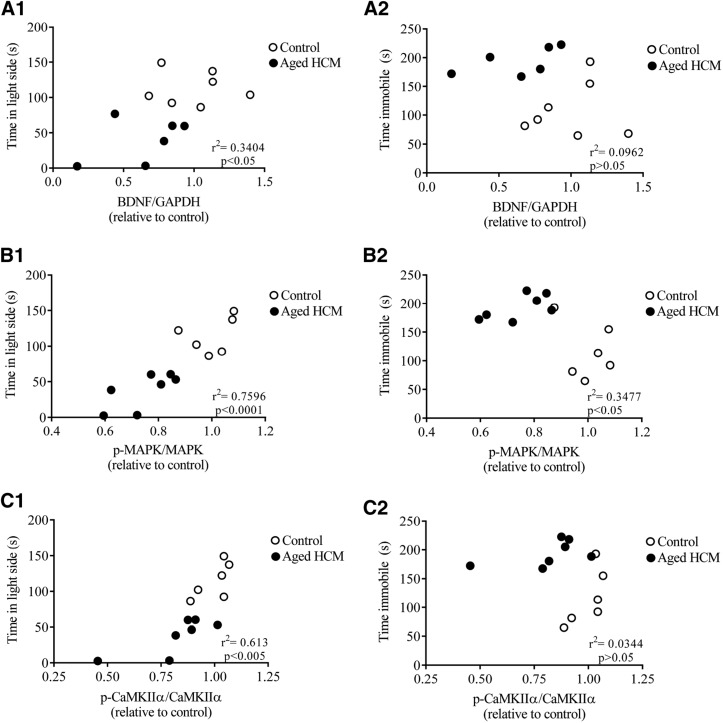
In young adult mice, genotype did not influence HPC BDNF levels [t (10) = 0.861; P > 0.05], consistent with the results from the behavioral assays. In contrast, aged HCM adults exhibited lower BDNF levels vs. controls [t (11) = 2.431; P < 0.05; Fig. 7A], consistent with the phenotype exhibited by aged HCM females. There was a significant correlation between anxiety-like behavior and BDNF levels (r2 = 0.3404; P < 0.05; Fig. 8A1], but not for depression-like behavior (r2 = 0.09615; P > 0.05; Fig. 8A2).
Figure 7.
Markers of neurotrophic health and neuroplasticity are significantly downregulated in the HPC of aged adult HCM females. A) BNDF (14 kDa) levels over GAPDH (37 kDa). B) Levels of p-MAPK over MAPK (42/44 kDa). C) p-CaMKIIα over CaMKIIα (50 kDa) in young adult and aged mice. Data are means ± sem and expressed as a ratio of controls (within each respective age group) (n = 6–7/group). *P < 0.05 vs. control, aged adults. Insets: representative Western blots.
Figure 8.
Markers of neurotrophic health and neuroplasticity correlate with behavioral indices of mood disorder in aged adult females. A1–C1) Correlation between anxiety-like behavior in the light–dark box and BDNF/GAPDH (A1), p-MAPK/MAPK (B1), and p-CaMKIIα/CaMKIIα levels in aged adult control and HCM mice (C1). A2–C2)Correlation between depression-like behavior in the FST and BDNF/GAPH (A2), p-MAPK/MAPK (B2), and p-CaMKIIα/CaMKIIα levels in the HPC of aged adult control and HCM mice (C2). Data are means ± sem and are expressed as a ratio of aged controls (n = 13/group).
Genotype exerted no significant effect on HPC levels of p-MAPK/MAPK in young adults [t (11) = 0.513; P > 0.05], whereas aged HCM adults exhibited lower HPC p-MAPK levels vs. controls [t (11) = 4.749; P < 0.001; Fig. 7B]. Consistent with the behavioral indices of mood disorder, there was a significant correlation between p-MAPK/MAPK and anxiety-like (r2 = 0.7596; P < 0.0001; Fig. 8B1) and depression-like (r2 = 0.3477; P < 0.05; Fig. 8B2) behaviors.
There was no significant effect of genotype in young adults, to influence levels of p-CaMKIIα/CaMKIIα [t (11) = 0.069; P > 0.05]. In contrast, aged HCM adults exhibited lower HPC p-CaMKIIα/CaMKIIα than the aged controls [t (11) = 2.272; P < 0.05; Fig. 7C]. We observed a significant relationship between anxiety-like behavior and p-CaMKIIα/CaMKIIα (r2 = 0.613; P < 0.005; Fig. 8C1), but no relationship with depression-like behavior (r2 = 0.03437; P > 0.05; Fig. 8C2).
DISCUSSION
Delineating the mechanisms underlying the coexpression of cardiovascular dysfunction and mood disorder has proven to be a challenge. This is due to the heterogeneous etiology of each disorder and the range of symptoms expressed by individuals. The coexpression of these conditions is a serious concern for affected individuals, because mood disorder can increase morbidity and mortality from heart disease (23, 24, 26–28). The overarching goal of the present study was to identify whether a mouse model of genetically induced HCM can replicate the core behavioral features of mood disorder, as is observed in the human population. A genetic mouse model of HCM has several advantages over other rodent models of cardiovascular disease (e.g., coronary artery ligation). The first advantage is that the cTnC A8V mutation is chronically expressed and captures the long-term effects of this disease, as has been observed in the human population. In addition, this genetic mouse model does not undergo surgical stress/chemical exposures that are present in other models of heart disease (e.g., coronary artery ligation), and the cardiac-specific knock-in gene is the only factor that differs between control and HCM mice. Although there is no inherent advantage to using a mouse expressing the cTnC A8V mutation over other HCM-related mutations (e.g., myosin binding protein C), our mouse model displays a cardiac phenotype consistent with HCM (9, 13–16).
It is well-established that females have a 2-fold increased prevalence of anxiety and depression when compared to men (39). This finding has also been documented in a population with cardiovascular disorders, identifying females as an especially vulnerable population (22, 40, 41). As such, we focused on females in the present study and intend to examine males as well in future studies to determine whether there are sex differences in the comorbidity between heart disease and mood disorders. Examination of this mouse model of HCM revealed that aging of the animal (i.e., duration of disease state) was a key contributor to pathophysiological features of stress, behavioral indices of anxiety and depression, and neural characteristics of mood disorder. Furthermore, we believe that these pathophysiological features of stress/mood disorder developed in response to the cardiac-specific HCM mutation.
An additional goal of our study was to determine whether female mice exhibited echo features of HCM at the same ages and during the same developmental periods. Young and aged adults displayed echo evidence of HCM, yet we identified some age differences in disease severity. For example, aged HCM females exhibited impairments in IVCT and IVRT, which is indicative of diastolic dysfunction. In contrast, young adults exhibited normal IVCT and a trend toward impairments in IVRT.
Echo and EEG confirmation of HCM phenotype
In the present study, echo confirmed HCM characteristics in young adult females. However, these mice did not differ from age-matched controls in expression of anxiety- and depression-like behavior. Conversely, aged HCM adults exhibited an anxiety-like phenotype in the light–dark box and higher levels of behavioral despair in the FST, compared with age-matched controls. These differences in indices of anxiety and depression are not the result of impaired locomotor activity, because both HCM and control mice made a similar number of transitions between the compartments of the light–dark box. The mood disorder-like phenotype exhibited by aged HCM adults prompted us investigate peripheral and central mechanisms that may be sensitive to HCM and promote this mood disorder-like phenotype.
We used ECG to investigate the beat-to-beat electrical activity of the heart and its autonomic modulation. Consistent with the echo aged HCM adults exhibited ECG characteristics of HCM. These changes include lower RMSSD, reduced CV, and diminished HRV, which are indicative of a sympathetic–dominant autonomic tone (60). It is important to note, HRV serves as an indicator of heart function, as well as a gauge of the severity of depression (65). As such, we examined the relationship between RMSSD and anxiety- and depression-like behavior; we focused on this ECG parameter because it is a reflection of the parasympathetic input to the heart (63, 64). We found that low RMSSD (i.e., high sympathetic input) correlated with levels of anxiety-like behavior in the light–dark box test and with behavioral despair in the FST. Activation of the SNS promotes behavioral responses to acute stressors and maintains homeostasis. However, prolonged SNS activation can exert stress on the heart and other SNS-sensitive organs (66). The adrenal gland is one organ that is sensitive to SNS activity (67), and it develops hypertrophy in response to chronic SNS stimulation (67, 68) and in response to HPA axis overactivation (69). Consistent with the ECG evidence of a predominant SNS tone, aged adult HCM females displayed adrenal gland hypertrophy as compared to age-matched controls. The behavioral and pathophysiological characteristics of these aged HCM adults are in line with clinical reports of individuals with depression (70, 71).
Peripheral indicators of cardiovascular distress and mood disorder
Despite developing adrenal gland hypertrophy, assay of plasma Cort revealed that HCM status did not influence Cort levels. This finding was unexpected because of the many reports of increased HPA axis activity in animal models of, and in humans with, depression (72, 73). Because we collected blood early in the light phase when Cort levels are naturally low (74), the effect of HCM on Cort may have been masked. It is also possible that, over time, the HPA axis of HCM mice habituates, in an effort to protect the organism from excessive Cort stimulation (75). If this habituation occurred, aged adult HCM females may exhibit hypersensitive Cort responses if subjected to a novel stressor before blood collection (69). Future studies should examine stress-induced Cort responses in aged HCM mice to fully assess HPA axis activity.
Another pathophysiological feature shared by HCM and mood disorder is an activated immune system. The immune system promotes the release of the proinflammatory cytokines, such as IL-6, which is found at higher levels in patients with depression (76) and in those with HCM (77). In the present study we detected no HCM-associated changes in IL-6 levels; consistent with a previous study conducted in mice with HCM (78), which may be related to species differences in physiologic responses to HCM. However, it is important to note that other proinflammatory cytokines, such as IL-1β or IL-10 (79, 80), may be elevated in response to the physiologic stress associated with our mouse model of HCM.
Neural correlates of cardiovascular distress and mood disorder
With this knowledge in hand, we examined HCM-associated changes within the brain. Brain imaging and functional studies of major depressive disorder have identified reductions in gray matter, reduced volume, and impaired tissue integrity of the PFC (81), CPu (82, 83), and HPC (52). We used structural MRI to investigate whether aged HCM adult mice expressed volumetric changes that correspond with the behavioral ramifications associated with HCM. We found that PFC and CPu volume were significantly reduced in aged HCM females compared with controls, and we detected a trend toward volume reduction within the HPC. Our findings are congruent with human studies, which describe volumetric changes in mood-related brain regions in patients with heart failure comorbid with depression and anxiety (84–88).
In addition to volumetric changes, impairments in neurogenesis and neuroplasticity have been implicated in the pathophysiology of mood disorder (89, 90). As such, we probed for these established molecular changes in the HPC. The neurotrophic family member, BDNF, exerts its effect by binding to the tropomyosin receptor kinase B, which then initiates a cascade of intracellular signaling events that can promote the activation of transcription factors and gene expression to support neuronal plasticity (53, 56). Young HCM female mice displayed no differences in BDNF levels, as compared to age-matched controls. We identified lower levels of BDNF within the HPC of aged HCM mice vs. controls, and BDNF levels correlated significantly with anxiety-like behavior. This finding is consistent with previous reports that chronic stress induces anxiety-like behavior in parallel with the down-regulation of BDNF levels in the HPC (91).
A key intracellular signaling cascade influenced by BDNF is the MAPK pathway (55). p-MAPK impinges upon downstream targets to promote the survival and health of neurons via gene transcription and promotes subsequent BDNF expression (92). Young adult females displayed no HCM-related changes in this molecular marker of plasticity. Yet, we identified a significant down-regulation of p-MAPK in aged HCM mice as compared to age-matched controls. We also detected a correlation between levels of p-MAPK and expression of anxiety- and depression-like behavior. These results are consistent with reports of reduced p-MAPK in suicide victims and in rodents subjected to chronic mild stress (93) and are in line with evidence that inhibitors of the MAPK pathway increase behavioral despair in the FST (94).
CaMKIIα is another intracellular signaling molecule that plays a role in HPC long-term potentiation and is critical for initiating many biochemical cascades that promote neuroplasticity (95, 96). Stress negatively impacts levels of intracellular Ca2+ (97), and stress-related Ca2+ loss leads to reductions in levels of activated CaMKIIα (98). In direct contrast to the effects of stress, antidepressant compounds increase p-CaMKIIα levels (99). Consistent with our behavioral results, young adults displayed no effect of HCM on this marker of neuroplasticity, as compared to controls. Yet, aged HCM adults exhibited significantly lower p-CaMKIIα levels as compared to controls; and levels of p-CaMKIIα correlated significantly with anxiety-like behaviors. Our results are consistent with evidence that impaired hemodynamics are associated with maladaptive brain aging (100); which together indicate that HCM-associated changes in heart function may influence neurochemical and neuroimaging markers of mood disorder.
CONCLUSIONS
We describe a mouse model of HCM where females exhibit indices of mood disorder that fulfill many of the criteria for an animal model of a human disorder (101). One shortcoming of the present investigation was the use of only female HCM mice. In humans and in rodent studies of heart disease, males often exhibit more symptoms of disease as compared to females (102–104), and estrogen appears to play a cardioprotective role in females (105, 106). Future investigations should examine the impact of TnC A8V and other sarcomeric mutations in males and females to understand how genetic sex can influence cardiovascular pathophysiology and development of a mood disorder–like phenotype.
In our study, HCM-associated behavioral, MRI volumetric, and neurochemical indices of mood disorder were observed in only aged HCM females and suggests that prolonged systemic stress of HCM is central to the development of a mood disorder. We suggest that sarcomeric mutations with higher prevalence in the human population (e.g., myosin binding protein C and myosin heavy chain) may exert similar pathologic changes in patients with mood disorder, and these mutations should be explored for mood disorder–like symptoms in mouse models. In addition, we posit that these same pathologic changes would also be observed in patients who experience mood disorder in concert with other forms of heart failure, because these conditions share features with HCM (e.g., SNS overactivation). If these mood disorder symptoms are left untreated, they may worsen the prognosis of patients with HCM. In support of this possibility, studies have shown that antidepressant treatment reduces mortality of individuals with depression and heart disease (107, 108). For individuals who cannot or do not want to take pharmacotherapies for depression, nonpharmacologic treatments such as talk therapy and stress management have been shown to be effective (109). Taken together, our results reinforce the notion that in addition to treating cardiovascular symptoms, clinicians should assess the presence of mood disorder and treat both conditions to improve their patients’ quality of life.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by U.S. National Institutes of Health, Heart, Lung, and Blood Institute Grants HL103840 and HL128683 (to J.R.P.). The authors declare no conflicts of interest.
Glossary
- AET
aortic ejection time
- BDNF
brain-derived neurotrophic factor
- CaMKIIα
Ca2+/calmodulin-dependent protein kinase II α
- Cort
corticosterone
- CPu
caudate putamen
- cTn
cardiac troponin
- CV
coefficient of variation
- ECG
electrocardiography
- echo
echocardiography
- EF
ejection fraction
- FS
fractional shortening
- FST
forced swim test
- HCM
hypertrophic cardiomyopathy
- HF
high frequency
- HPC
hippocampus
- HR
heart rate
- HRV
heart rate variance
- IV
isovolumetric
- IVCT
IV contraction time
- IVRT
IV relaxation time
- LF
low frequency
- LSD
least significant difference
- LV MPI IV
left ventricle myocardial performance IV
- LV MPI NFT
left ventricle myocardial performance nonfilling time
- MV
mitral valve
- MV A
mitral valve atrial late peak flow velocity
- MV E
mitral valve early peak flow velocity
- OD
optical density
- PFA
paraformaldehyde
- PFC
prefrontal cortex
- R–R
R-wave to R-wave
- RMSSD
root-mean square of the successive difference
- SNS
sympathetic nervous system
- TP
total power
- TBS
Tris-buffered saline
- TBS-T
TBS-Tween
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. M. Dossat, M. Sanchez-Gonzalez, J. R. Pinto, and M. Kabbaj designed the research; A. M. Dossat, M. A. Sanchez-Gonzalez, A. Koutnik, S. Leitner, E. Ruiz, and B. Griffin performed research; A. M. Dossat and M. A. Sanchez-Gonzalez analyzed data; A. M. Dossat, J. R. Pinto, and M. Kabbaj wrote the manuscript; J. T. Rosenberg and S. C. Grant performed volumetric analysis of the brain; and F. D. Fincham helped in editing the manuscript.
REFERENCES
- 1.Force T., Bonow R. O., Houser S. R., Solaro R. J., Hershberger R. E., Adhikari B., Anderson M. E., Boineau R., Byrne B. J., Cappola T. P., Kalluri R., LeWinter M. M., Maron M. S., Molkentin J. D., Ommen S. R., Regnier M., Tang W. H., Tian R., Konstam M. A., Maron B. J., Seidman C. E. (2010) Research priorities in hypertrophic cardiomyopathy: report of a Working Group of the National Heart, Lung, and Blood Institute. Circulation 122, 1130–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fatkin D., Seidman C. E., Seidman J. G. (2014) Genetics and disease of ventricular muscle. Cold Spring Harb. Perspect. Med. 4, a021063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113, 1807–1816 [DOI] [PubMed] [Google Scholar]
- 4.Semsarian C., Ingles J., Maron M. S., Maron B. J. (2015) New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 65, 1249–1254 [DOI] [PubMed] [Google Scholar]
- 5.Morita H., Rehm H. L., Menesses A., McDonough B., Roberts A. E., Kucherlapati R., Towbin J. A., Seidman J. G., Seidman C. E. (2008) Shared genetic causes of cardiac hypertrophy in children and adults. N. Engl. J. Med. 358, 1899–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidman C. E., Seidman J. G. (2011) Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ. Res. 108, 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willott R. H., Gomes A. V., Chang A. N., Parvatiyar M. S., Pinto J. R., Potter J. D. (2010) Mutations in troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J. Mol. Cell. Cardiol. 48, 882–892 [DOI] [PubMed] [Google Scholar]
- 8.Harvey P. A., Leinwand L. A. (2011) The cell biology of disease: cellular mechanisms of cardiomyopathy. J. Cell Biol. 194, 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins A. S., Parvatiyar M. S., Feng H. Z., Bos J. M., Gonzalez-Martinez D., Vukmirovic M., Turna R. S., Sanchez-Gonzalez M. A., Badger C. D., Zorio D. A., Singh R. K., Wang Y., Jin J. P., Ackerman M. J., Pinto J. R. (2015) In vivo analysis of troponin C knock-in (A8V) mice: evidence that TNNC1 is a hypertrophic cardiomyopathy susceptibility gene. Circ Cardiovasc Genet 8, 653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landstrom A. P., Parvatiyar M. S., Pinto J. R., Marquardt M. L., Bos J. M., Tester D. J., Ommen S. R., Potter J. D., Ackerman M. J. (2008) Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. J. Mol. Cell. Cardiol. 45, 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaafar N., Girolami F., Zairi I., Kraiem S., Hammami M., Olivotto I. (2015) Genetic profile of hypertrophic cardiomyopathy in Tunisia: Is it different? Glob. Cardiol. Sci. Pract. 2015, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ploski R., Rydzanicz M., Ksiazczyk T. M., Franaszczyk M., Pollak A., Kosinska J., Michalak E., Stawinski P., Ziolkowska L., Bilinska Z. T., Werner B. (2016) Evidence for troponin C (TNNC1) as a gene for autosomal recessive restrictive cardiomyopathy with fatal outcome in infancy. Am. J. Med. Genet. A. 12, 3241–3248 [DOI] [PubMed] [Google Scholar]
- 13.Tardiff J. C., Hewett T. E., Palmer B. M., Olsson C., Factor S. M., Moore R. L., Robbins J., Leinwand L. A. (1999) Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J. Clin. Invest. 104, 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James J., Zhang Y., Osinska H., Sanbe A., Klevitsky R., Hewett T. E., Robbins J. (2000) Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ. Res. 87, 805–811 [DOI] [PubMed] [Google Scholar]
- 15.Knollmann B. C., Blatt S. A., Horton K., de Freitas F., Miller T., Bell M., Housmans P. R., Weissman N. J., Morad M., Potter J. D. (2001) Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J. Biol. Chem. 276, 10039–10048 [DOI] [PubMed] [Google Scholar]
- 16.Westermann D., Knollmann B. C., Steendijk P., Rutschow S., Riad A., Pauschinger M., Potter J. D., Schultheiss H. P., Tschöpe C. (2006) Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur. J. Heart Fail. 8, 115–121 [DOI] [PubMed] [Google Scholar]
- 17.Morgan J. F., O’Donoghue A. C., McKenna W. J., Schmidt M. M. (2008) Psychiatric disorders in hypertrophic cardiomyopathy. Gen. Hosp. Psychiatry 30, 49–54 [DOI] [PubMed] [Google Scholar]
- 18.Igoumenou A., Alevizopoulos G., Anastasakis A., Stavrakaki E., Toutouzas P., Stefanadis C. (2012) Depression in patients with hypertrophic cardiomyopathy: is there any relation with the risk factors for sudden death? Heart Asia 4, 44–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox S., O’Donoghue A. C., McKenna W. J., Steptoe A. (1997) Health related quality of life and psychological wellbeing in patients with hypertrophic cardiomyopathy. Heart 78, 182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingles J., Lind J. M., Phongsavan P., Semsarian C. (2008) Psychosocial impact of specialized cardiac genetic clinics for hypertrophic cardiomyopathy. Genet. Med. 10, 117–120 [DOI] [PubMed] [Google Scholar]
- 21.Mastrogiannis D., Giamouzis G., Dardiotis E., Karayannis G., Chroub-Papavaiou A., Kremeti D., Spiliopoulos K., Georgoulias P., Koutsias S., Bonotis K., Mantzorou M., Skoularigis J., Hadjigeorgiou G. M., Butler J., Triposkiadis F. (2012) Depression in patients with cardiovascular disease. Cardiol. Res. Pract. 2012, 794762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutledge T., Reis V. A., Linke S. E., Greenberg B. H., Mills P. J. (2006) Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J. Am. Coll. Cardiol. 48, 1527–1537 [DOI] [PubMed] [Google Scholar]
- 23.Moussavi S., Chatterji S., Verdes E., Tandon A., Patel V., Ustun B. (2007) Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370, 851–858 [DOI] [PubMed] [Google Scholar]
- 24.Scott K. M., Bruffaerts R., Tsang A., Ormel J., Alonso J., Angermeyer M. C., Benjet C., Bromet E., de Girolamo G., de Graaf R., Gasquet I., Gureje O., Haro J. M., He Y., Kessler R. C., Levinson D., Mneimneh Z. N., Oakley Browne M. A., Posada-Villa J., Stein D. J., Takeshima T., Von Korff M. (2007) Depression-anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys. J. Affect. Disord. 103, 113–120 [DOI] [PubMed] [Google Scholar]
- 25.Frasure-Smith N., Lespérance F., Talajic M. (1993) Depression following myocardial infarction: impact on 6-month survival. JAMA 270, 1819–1825 [PubMed] [Google Scholar]
- 26.Jiang W., Alexander J., Christopher E., Kuchibhatla M., Gaulden L. H., Cuffe M. S., Blazing M. A., Davenport C., Califf R. M., Krishnan R. R., O’Connor C. M. (2001) Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch. Intern. Med. 161, 1849–1856 [DOI] [PubMed] [Google Scholar]
- 27.Vaccarino V., Kasl S. V., Abramson J., Krumholz H. M. (2001) Depressive symptoms and risk of functional decline and death in patients with heart failure. J. Am. Coll. Cardiol. 38, 199–205 [DOI] [PubMed] [Google Scholar]
- 28.Lespérance F., Frasure-Smith N., Talajic M., Bourassa M. G. (2002) Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 105, 1049–1053 [DOI] [PubMed] [Google Scholar]
- 29.Ito K., Hirooka Y., Sunagawa K. (2013) Brain sigma-1 receptor stimulation improves mental disorder and cardiac function in mice with myocardial infarction. J. Cardiovasc. Pharmacol. 62, 222–228 [DOI] [PubMed] [Google Scholar]
- 30.Wann B. P., Bah T. M., Boucher M., Courtemanche J., Le Marec N., Rousseau G., Godbout R. (2007) Vulnerability for apoptosis in the limbic system after myocardial infarction in rats: a possible model for human postinfarct major depression. J. Psychiatry Neurosci. 32, 11–16 [PMC free article] [PubMed] [Google Scholar]
- 31.Frey A., Popp S., Post A., Langer S., Lehmann M., Hofmann U., Sirén A. L., Hommers L., Schmitt A., Strekalova T., Ertl G., Lesch K. P., Frantz S. (2014) Experimental heart failure causes depression-like behavior together with differential regulation of inflammatory and structural genes in the brain. Front. Behav. Neurosci. 8, 376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henze M., Tiniakov R., Samarel A., Holmes E., Scrogin K. (2013) Chronic fluoxetine reduces autonomic control of cardiac rhythms in rats with congestive heart failure. Am. J. Physiol. Heart Circ. Physiol. 304, H444–H454 [DOI] [PubMed] [Google Scholar]
- 33.Henze M., Hart D., Samarel A., Barakat J., Eckert L., Scrogin K. (2008) Persistent alterations in heart rate variability, baroreflex sensitivity, and anxiety-like behaviors during development of heart failure in the rat. Am. J. Physiol. Heart Circ. Physiol. 295, H29–H38 [DOI] [PubMed] [Google Scholar]
- 34.Wann B. P., Bah T. M., Kaloustian S., Boucher M., Dufort A. M., Le Marec N., Godbout R., Rousseau G. (2009) Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: effects of sertraline. J. Psychopharmacol. (Oxford) 23, 451–459 [DOI] [PubMed] [Google Scholar]
- 35.Wann B. P., Audet M. C., Anisman H. (2010) Impact of acute and chronic stressor experiences on heart atrial and brain natriuretic peptides in response to a subsequent stressor. Horm. Behav. 58, 907–916 [DOI] [PubMed] [Google Scholar]
- 36.Grippo A. J., Beltz T. G., Weiss R. M., Johnson A. K. (2006) The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol. Psychiatry 59, 309–316 [DOI] [PubMed] [Google Scholar]
- 37.Grippo A. J., Moffitt J. A., Sgoifo A., Jepson A. J., Bates S. L., Chandler D. L., McNeal N., Preihs K. (2012) The integration of depressive behaviors and cardiac dysfunction during an operational measure of depression: investigating the role of negative social experiences in an animal model. Psychosom. Med. 74, 612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sgoifo A., Carnevali L., Grippo A. J. (2014) The socially stressed heart. Insights from studies in rodents. Neurosci. Biobehav. Rev. 39, 51–60 [DOI] [PubMed] [Google Scholar]
- 39.Kessler R. C., Petukhova M., Sampson N. A., Zaslavsky A. M., Wittchen H. U. (2012) Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 21, 169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlieb S. S., Khatta M., Friedmann E., Einbinder L., Katzen S., Baker B., Marshall J., Minshall S., Robinson S., Fisher M. L., Potenza M., Sigler B., Baldwin C., Thomas S. A. (2004) The influence of age, gender, and race on the prevalence of depression in heart failure patients. J. Am. Coll. Cardiol. 43, 1542–1549 [DOI] [PubMed] [Google Scholar]
- 41.PREMIER Registry Investigators (2006) Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch. Intern. Med. 166, 876–883 [DOI] [PubMed] [Google Scholar]
- 42.Ouyang P., Wenger N. K., Taylor D., Rich-Edwards J. W., Steiner M., Shaw L. J., Berga S. L., Miller V. M., Merz N. B. (2016) Strategies and methods to study female-specific cardiovascular health and disease: a guide for clinical scientists. Biol. Sex Differ. 7, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das U. N. (2000) Free radicals, cytokines and nitric oxide in cardiac failure and myocardial infarction. Mol. Cell. Biochem. 215, 145–152 [DOI] [PubMed] [Google Scholar]
- 44.Dunn A. J., Swiergiel A. H., de Beaurepaire R. (2005) Cytokines as mediators of depression: what can we learn from animal studies? Neurosci. Biobehav. Rev. 29, 891–909 [DOI] [PubMed] [Google Scholar]
- 45.McEwen B. S. (2004) Structural plasticity of the adult brain: how animal models help us understand brain changes in depression and systemic disorders related to depression. Dialogues Clin. Neurosci. 6, 119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhebergen D., Korten N. C., Penninx B. W., Stek M. L., van der Mast R. C., Oude Voshaar R., Comijs H. C. (2015) Hypothalamic-pituitary-adrenal axis activity in older persons with and without a depressive disorder. Psychoneuroendocrinology 51, 341–350 [DOI] [PubMed] [Google Scholar]
- 47.Golkar A., Lonsdorf T. B., Olsson A., Lindstrom K. M., Berrebi J., Fransson P., Schalling M., Ingvar M., Öhman A. (2012) Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One 7, e48107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russo S. J., Nestler E. J. (2013) The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grieve S. M., Korgaonkar M. S., Koslow S. H., Gordon E., Williams L. M. (2013) Widespread reductions in gray matter volume in depression. Neuroimage Clin. 3, 332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IMAGe Research Group (2009) Three-dimensional surface mapping of the caudate nucleus in late-life depression. Am. J. Geriatr. Psychiatry 17, 4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo M. A., Kumar R., Macey P. M., Fonarow G. C., Harper R. M. (2009) Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J. Card. Fail. 15, 214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drevets W. C., Price J. L., Furey M. L. (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 213, 93–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barbacid M. (1995) Neurotrophic factors and their receptors. Curr. Opin. Cell Biol. 7, 148–155 [DOI] [PubMed] [Google Scholar]
- 54.Blanquet P. R., Mariani J., Derer P. (2003) A calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cyclic AMP-responsive transcription factor in the rat hippocampus. Neuroscience 118, 477–490 [DOI] [PubMed] [Google Scholar]
- 55.Reichardt L. F. (2006) Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thoenen H. (2000) Neurotrophins and activity-dependent plasticity. Prog. Brain Res. 128, 183–191 [DOI] [PubMed] [Google Scholar]
- 57.Dweck D., Sanchez-Gonzalez M. A., Chang A. N., Dulce R. A., Badger C. D., Koutnik A. P., Ruiz E. L., Griffin B., Liang J., Kabbaj M., Fincham F. D., Hare J. M., Overton J. M., Pinto J. R. (2014) Long term ablation of protein kinase A (PKA)-mediated cardiac troponin I phosphorylation leads to excitation-contraction uncoupling and diastolic dysfunction in a knock-in mouse model of hypertrophic cardiomyopathy. J. Biol. Chem. 289, 23097–23111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh J. K., Hatle L., Tajik A. J., Little W. C. (2006) Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J. Am. Coll. Cardiol. 47, 500–506 [DOI] [PubMed] [Google Scholar]
- 59.Thireau J., Zhang B. L., Poisson D., Babuty D. (2008) Heart rate variability in mice: a theoretical and practical guide. Exp. Physiol. 93, 83–94 [DOI] [PubMed] [Google Scholar]
- 60.Rahman A., Liu D. (2011) Pericarditis: clinical features and management. Aust. Fam. Physician 40, 791–796 [PubMed] [Google Scholar]
- 61.Porsolt R. D., Bertin A., Jalfre M. (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 229, 327–336 [PubMed] [Google Scholar]
- 62.Paxinos G., Franklin K. B. J. (2012) The Mouse Brain in Stereotaxic Coordinates, Academic Press, San Diego, CA [Google Scholar]
- 63.Stein P. K., Bosner M. S., Kleiger R. E., Conger B. M. (1994) Heart rate variability: a measure of cardiac autonomic tone. Am. Heart J. 127, 1376–1381 [DOI] [PubMed] [Google Scholar]
- 64.Sgoifo A., de Boer S. F., Westenbroek C., Maes F. W., Beldhuis H., Suzuki T., Koolhaas J. M. (1997) Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am. J. Physiol. 273, H1754–H1760 [DOI] [PubMed] [Google Scholar]
- 65.Kemp A. H., Quintana D. S., Gray M. A., Felmingham K. L., Brown K., Gatt J. M. (2010) Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry 67, 1067–1074 [DOI] [PubMed] [Google Scholar]
- 66.Florea V. G., Cohn J. N. (2014) The autonomic nervous system and heart failure. Circ. Res. 114, 1815–1826 [DOI] [PubMed] [Google Scholar]
- 67.Tank A. W., Lee Wong D. (2015) Peripheral and central effects of circulating catecholamines. Compr. Physiol. 5, 1–15 [DOI] [PubMed] [Google Scholar]
- 68.Schneider B., Prvulovic D., Oertel-Knöchel V., Knöchel C., Reinke B., Grexa M., Weber B., Hampel H. (2011) Biomarkers for major depression and its delineation from neurodegenerative disorders. Prog. Neurobiol. 95, 703–717 [DOI] [PubMed] [Google Scholar]
- 69.De Kloet E. R., Joëls M., Holsboer F. (2005) Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475 [DOI] [PubMed] [Google Scholar]
- 70.Nemeroff C. B., Krishnan K. R., Reed D., Leder R., Beam C., Dunnick N. R. (1992) Adrenal gland enlargement in major depression: a computed tomographic study. Arch. Gen. Psychiatry 49, 384–387 [DOI] [PubMed] [Google Scholar]
- 71.Rubin R. T., Phillips J. J., Sadow T. F., McCracken J. T. (1995) Adrenal gland volume in major depression. Increase during the depressive episode and decrease with successful treatment. Arch. Gen. Psychiatry 52, 213–218 [DOI] [PubMed] [Google Scholar]
- 72.Pariante C. M., Lightman S. L. (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 31, 464–468 [DOI] [PubMed] [Google Scholar]
- 73.Hollis F., Kabbaj M. (2014) Social defeat as an animal model for depression. ILAR J. 55, 221–232 [DOI] [PubMed] [Google Scholar]
- 74.Zimmermann E., Critchlow V. (1967) Effects of diurnal variation in plasma corticosterone levels on adrenocortical response to stress. Proc. Soc. Exp. Biol. Med. 125, 658–663 [DOI] [PubMed] [Google Scholar]
- 75.Girotti M., Pace T. W., Gaylord R. I., Rubin B. A., Herman J. P., Spencer R. L. (2006) Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience 138, 1067–1081 [DOI] [PubMed] [Google Scholar]
- 76.Miller A. H., Raison C. L. (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuusisto J., Kärjä V., Sipola P., Kholová I., Peuhkurinen K., Jääskeläinen P., Naukkarinen A., Ylä-Herttuala S., Punnonen K., Laakso M. (2012) Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart 98, 1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magida J. A., Leinwand L. A. (2014) Metabolic crosstalk between the heart and liver impacts familial hypertrophic cardiomyopathy. EMBO Mol. Med. 6, 482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parissis J. T., Adamopoulos S., Rigas A., Kostakis G., Karatzas D., Venetsanou K., Kremastinos D. T. (2004) Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with vs. without symptoms of depression. Am. J. Cardiol. 94, 1326–1328 [DOI] [PubMed] [Google Scholar]
- 80.Bay-Richter C., Hallberg L., Ventorp F., Janelidze S., Brundin L. (2012) Aldosterone synergizes with peripheral inflammation to induce brain IL-1β expression and depressive-like effects. Cytokine 60, 749–754 [DOI] [PubMed] [Google Scholar]
- 81.Drevets W. C. (2007) Orbitofrontal cortex function and structure in depression. Ann. N. Y. Acad. Sci. 1121, 499–527 [DOI] [PubMed] [Google Scholar]
- 82.Husain M. M., McDonald W. M., Doraiswamy P. M., Figiel G. S., Na C., Escalona P. R., Boyko O. B., Nemeroff C. B., Krishnan K. R. (1991) A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 40, 95–99 [DOI] [PubMed] [Google Scholar]
- 83.Krishnan K. R., McDonald W. M., Escalona P. R., Doraiswamy P. M., Na C., Husain M. M., Figiel G. S., Boyko O. B., Ellinwood E. H., Nemeroff C. B. (1992) Magnetic resonance imaging of the caudate nuclei in depression: preliminary observations. Arch. Gen. Psychiatry 49, 553–557 [DOI] [PubMed] [Google Scholar]
- 84.Agid R., Levin T., Gomori J. M., Lerer B., Bonne O. (2003) T2-weighted image hyperintensities in major depression: focus on the basal ganglia. Int. J. Neuropsychopharmacol. 6, 215–224 [DOI] [PubMed] [Google Scholar]
- 85.Almeida O. P., Garrido G. J., Etherton-Beer C., Lautenschlager N. T., Arnolda L., Alfonso H., Flicker L. (2013) Brain and mood changes over 2 years in healthy controls and adults with heart failure and ischaemic heart disease. Eur. J. Heart Fail. 15, 850–858 [DOI] [PubMed] [Google Scholar]
- 86.Kumar R., Nguyen H. D., Ogren J. A., Macey P. M., Thompson P. M., Fonarow G. C., Hamilton M. A., Harper R. M., Woo M. A. (2011) Global and regional putamen volume loss in patients with heart failure. Eur. J. Heart Fail. 13, 651–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woo M. A., Ogren J. A., Abouzeid C. M., Macey P. M., Sairafian K. G., Saharan P. S., Thompson P. M., Fonarow G. C., Hamilton M. A., Harper R. M., Kumar R. (2015) Regional hippocampal damage in heart failure. Eur. J. Heart Fail. 17, 494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woo M. A., Palomares J. A., Macey P. M., Fonarow G. C., Harper R. M., Kumar R. (2015) Global and regional brain mean diffusivity changes in patients with heart failure. J. Neurosci. Res. 93, 678–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith M. A., Makino S., Kvetnansky R., Post R. M. (1995) Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 15, 1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.David D. J., Samuels B. A., Rainer Q., Wang J. W., Marsteller D., Mendez I., Drew M., Craig D. A., Guiard B. P., Guilloux J. P., Artymyshyn R. P., Gardier A. M., Gerald C., Antonijevic I. A., Leonardo E. D., Hen R. (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62, 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu W. X., Wang J., Xie Z. M., Xu N., Zhang G. F., Jia M., Zhou Z. Q., Hashimoto K., Yang J. J. (2016) Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology (Berl.) 233, 405–415 [DOI] [PubMed] [Google Scholar]
- 92.Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153–183 [DOI] [PubMed] [Google Scholar]
- 93.Duric V., Banasr M., Licznerski P., Schmidt H. D., Stockmeier C. A., Simen A. A., Newton S. S., Duman R. S. (2010) A negative regulator of MAP kinase causes depressive behavior. Nat. Med. 16, 1328–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duman C. H., Schlesinger L., Kodama M., Russell D. S., Duman R. S. (2007) A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol. Psychiatry 61, 661–670 [DOI] [PubMed] [Google Scholar]
- 95.Erondu N. E., Kennedy M. B. (1985) Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci. 5, 3270–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayford M., Bach M. E., Huang Y. Y., Wang L., Hawkins R. D., Kandel E. R. (1996) Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678–1683 [DOI] [PubMed] [Google Scholar]
- 97.Kim J. J., Yoon K. S. (1998) Stress: metaplastic effects in the hippocampus. Trends Neurosci. 21, 505–509 [DOI] [PubMed] [Google Scholar]
- 98.Gerges N. Z., Aleisa A. M., Schwarz L. A., Alkadhi K. A. (2004) Reduced basal CaMKII levels in hippocampal CA1 region: possible cause of stress-induced impairment of LTP in chronically stressed rats. Hippocampus 14, 402–410 [DOI] [PubMed] [Google Scholar]
- 99.Tiraboschi E., Giambelli R., D’Urso G., Galietta A., Barbon A., de Bartolomeis A., Gennarelli M., Barlati S., Racagni G., Popoli M. (2004) Antidepressants activate CaMKII in neuron cell body by Thr286 phosphorylation. Neuroreport 15, 2393–2396 [DOI] [PubMed] [Google Scholar]
- 100.Jefferson A. L. (2010) Cardiac output as a potential risk factor for abnormal brain aging. J. Alzheimers Dis. 20, 813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Belzung C., Lemoine M. (2011) Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 1, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blenck C. L., Harvey P. A., Reckelhoff J. F., Leinwand L. A. (2016) The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ. Res. 118, 1294–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Olsson M. C., Palmer B. M., Leinwand L. A., Moore R. L. (2001) Gender and aging in a transgenic mouse model of hypertrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 280, H1136–H1144 [DOI] [PubMed] [Google Scholar]
- 104.Geisterfer-Lowrance A. A., Christe M., Conner D. A., Ingwall J. S., Schoen F. J., Seidman C. E., Seidman J. G. (1996) A mouse model of familial hypertrophic cardiomyopathy. Science 272, 731–734 [DOI] [PubMed] [Google Scholar]
- 105.Bhupathy P., Haines C. D., Leinwand L. A. (2010) Influence of sex hormones and phytoestrogens on heart disease in men and women. Womens Health (Lond) 6, 77–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Menazza S., Murphy E. (2016) The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ. Res. 118, 994–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krivoy A., Balicer R. D., Feldman B., Hoshen M., Zalsman G., Weizman A., Shoval G. (2015) Adherence to antidepressant therapy and mortality rates in ischaemic heart disease: cohort study. Br. J. Psychiatry 206, 297–301 [DOI] [PubMed] [Google Scholar]
- 108.Coupland C., Hill T., Morriss R., Moore M., Arthur A., Hippisley-Cox J. (2016) Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: cohort study using primary care database. BMJ 352, i1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klainin-Yobas P., Ng S. H., Stephen P. D., Lau Y. (2016) Efficacy of psychosocial interventions on psychological outcomes among people with cardiovascular diseases: a systematic review and meta-analysis. Patient Educ. Couns. 99, 512–521 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.