Figure 5.
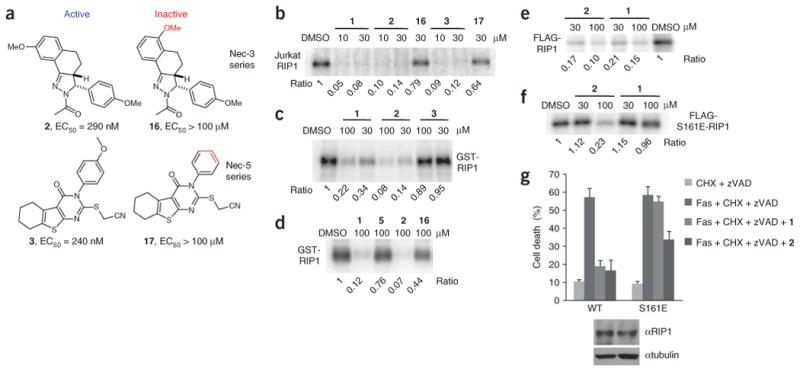
Necrostatin-3 and necrostatin-5 inhibit RIP1 kinase activity. (a) Structures of necrostatin-3 and necrostatin-5 analogs. Cellular EC50 in TNFα-treated FADD-deficient Jurkat cells are shown. (b) Additional Necs inhibit activity of Jurkat cell RIP1. In vitro kinase reaction in the presence of the indicated concentrations of necrostatins was performed as in Figure 1e. (c,d) 2, but not 16, specifically inhibits recombinant RIP1 kinase, expressed in Sf9 cells. In vitro kinase reactions in the presence of the indicated concentrations of necrostatins were performed as in Figure 2d. (e,f) 2 still inhibits activity of S161E mutant of RIP1 in vitro. WT FLAG-RIP1 (e) and S161E FLAG-RIP1 (f) were expressed in 293T cells and subjected to in vitro kinase assay in the presence of the indicated concentrations of necrostatins as in Figure 1c. Assays were performed at least two or three times, and similar results were obtained each time. The representative images are shown. (g) 2 attenuates necroptosis mediated by S161E mutant of RIP1. Recapitulation of WT and S161E mutants in RIP-deficient Jurkat cells and necroptosis viability assay were performed as described in Figure 3e. Compounds were used at 30 μM. The data were obtained in a single experiment performed in triplicate and represent mean values ± s.d. Experiment was repeated multiple times, and similar results were obtained each time. Equal expression of RIP1 mutants was confirmed by western blot using anti-RIP1 and anti-tubulin antibodies.
