Figure 4.
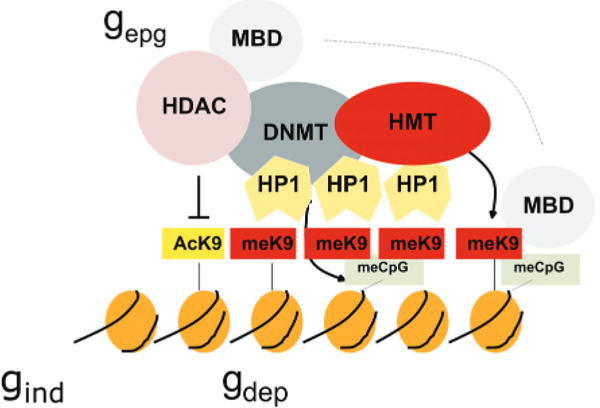
Cooperative and self-reinforcing organization of the chromatin- and DNA-modifying machinery responsible for gene silencing in normal and malignant cells. Histone (H3) modifications include lysine (K) acetylation (Ac) and lysine methylation (Me). Lysines at other positions are also modified. The HP1 protein recognizes MeK9 and, as this protein also binds the histone methyltransferase (HMT), heterochromatin can spread. Histone deacetylases (HDAC) deacetylate lysine residues as a prerequisite for their subsequent methylation. DNA methyltransferases (DNMT) participate in multiprotein complexes that contain HDACs and HMTs, and methyl-C binding proteins (MBD) can be loaded onto methylated DNA through their interactions with both HDACs and HMTs. Much of the evidence comes from studies of constitutive heterochromatin, but recent studies indicate similar interactions of genes silenced de novo in cancer cells. (Reprinted, with permission, from Feinberg and Tycko 2004 [©Nature Publishing Group; http://www.nature.com].)
