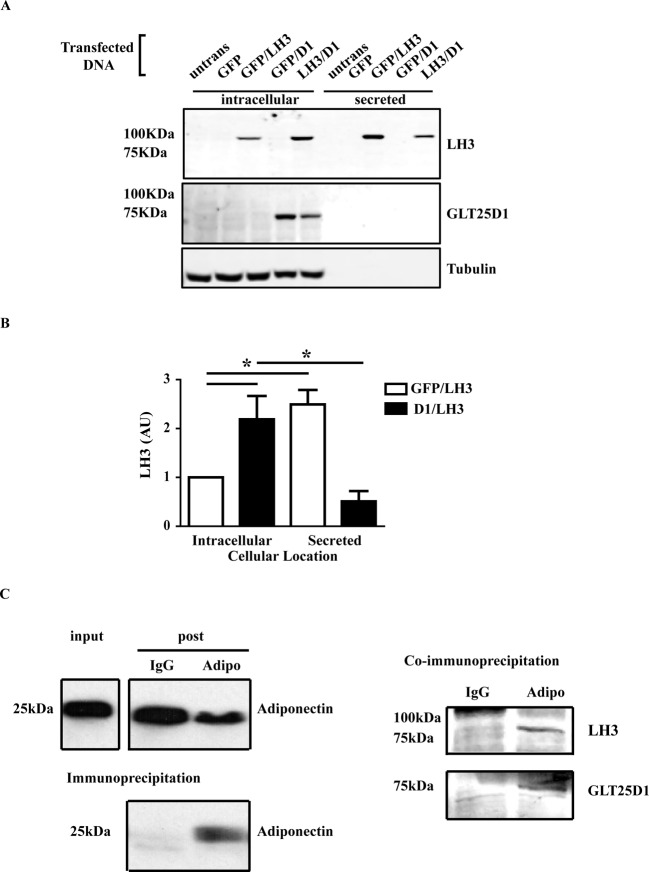
Figure 2. GLT25D1 interacts with LH3 and adiponectin.
Adipo-HEK cells were transiently expressed with LH3 or GLT25D1 or co-transfected together. GFP was used as filler DNA when only one enzyme was expressed. Cells were harvested 18 h post-transfection. 25 μg of protein lysate, and the proportional amount of media were reduced, boiled, separated on a Western blot and visualized using the Odyssey Infrared Imaging System. (A) LH3 was detected intracellularly and secreted. GLT25D1 was only detected intracellularly. β-Tubulin was used as a loading control. (B) Integrated intensity of the bands was used to quantitate the intracellular and secreted LH3. There was a significant increase in the intracellular LH3 when GLT25D1 was co-expressed (n=12, *P=0.0006, P<0.05). (C) SGBS cells were differentiated by the standard protocol until day 7. The media were replaced 24 h prior to co-immunoprecipitation with serum-free media containing 5 µM ascorbic acid. Adiponectin was immunoprecipitated using mouse anti-adiponectin antibody for 2 h at 4˚C. LH3 and GLT25D1 were analysed using the Odyssey Infrared Imaging System, and adiponectin was detected using enhanced chemiluminescence. Gamma was set at 1.0. Equal volumes and amounts of input and post-IP were loaded, equivalent to one-tenth of the IP. Adiponectin was pulled down in the presence of the mouse anti-adiponectin antibody, and was not pulled down by the IgG control. LH3 and GLT25D1 were co-immunoprecipitated with adiponectin (n=4).