Abstract
Background
In dogs with chronic valvular heart disease (CVHD), early recognition of pulmonary edema (PE) is of paramount importance. Recent studies in dogs showed that lung ultrasound examination (LUS) is a useful technique to diagnose cardiogenic PE.
Objectives
To describe LUS features in dogs with different stages of CVHD, and to determine its diagnostic accuracy in detecting PE using thoracic radiography as the reference standard.
Animals
Sixty‐three dogs with CVHD.
Methods
Prospective, multicenter, cross‐sectional study. Each dog underwent physical examination, echocardiography, thoracic radiography, and LUS. The LUS findings were classified as absent, rare, numerous, or confluent B‐lines. Sensitivity, specificity, and positive and negative predictive values of LUS B‐lines to identify PE were calculated using thoracic radiography as the reference standard.
Results
Dogs in stage B1 had absent or rare B‐lines in 14 of 15 cases (93.3%). Dogs in stage B2 had absent or rare B‐lines in 16 of 18 cases (88.9%). All dogs in stage C, without radiographic signs of PE, had absent or rare B‐lines. Dogs in stage C, with radiographic signs of PE, had numerous or confluent B‐lines in 18 of 20 cases (90%). Lung ultrasound examination detected PE with a sensitivity of 90%, specificity of 93%, and with positive and negative predictive values of 85.7 and 95.2%, respectively.
Conclusions and Clinical Importance
Lung ultrasound examination showed good diagnostic accuracy to identify cardiogenic PE and might be helpful in staging dogs with CVHD. Lung ultrasound examination should be considered as a new, noninvasive diagnostic tool for clinicians managing CVHD in dogs.
Keywords: Heart failure, Lung comets, Mitral valve, Pulmonary edema, Thoracic ultrasonography
Abbreviations
- CHF
congestive heart failure
- CVHD
chronic valvular heart disease
- E/A
E wave to A wave ratio of transmitral flow
- Emax
peak velocity of E wave of transmitral flow
- LA/Ao
left atrium aortic root ratio
- LUS
lung ultrasound
- LVIDDn
normalized left ventricular internal diameter in diastole
- PE
pulmonary edema
Chronic valvular heart disease (CVHD) is the most common acquired cardiac disease in dogs.1 The disease is characterized by a progressive degeneration of the mitral valve, which leads to mitral regurgitation. Mitral regurgitation can lead to cardiac remodeling and development of congestive heart failure (CHF). Although most dogs with CVHD remain asymptomatic for years, approximately one‐third develop CHF and die from their heart disease.2 Thus, both early recognition and prompt treatment of cardiac remodeling and CHF are of utmost clinical importance.3, 4, 5 Thoracic radiography is the most commonly used method for the diagnosis of cardiogenic pulmonary edema (PE) and currently is considered the clinical standard for diagnosis of PE in dogs.6, 7
In people, lung ultrasound examination (LUS) is used in the diagnosis of acute respiratory failure both in emergency medicine and cardiology.8, 9, 10 In people with acute heart failure, LUS is used to assess and stage PE with reliable results.11, 12, 13 In patients with PE, ultrasound identifies artifacts that appear as vertical hyperechoic lines with a narrow base that emerge from the surface of the pleura, extending to the distal edge of the screen, which are called B‐lines.14, 15, 16 These B‐lines correspond to the thickening of the subpleural and interlobular septa or to the presence of extravascular fluid in the lungs.12, 14 Studies in people indicate that the number and distribution of B‐lines correlate with pulmonary capillary wedge pressure, the presence of extravascular fluid in the lungs, and the severity of clinical presentation and prognosis.15, 17, 18, 19 Some patients with interstitial PE develop B‐lines before the onset of clinical or radiographic signs of heart failure; LUS may be of clinical utility in the early diagnosis of PE in these cases.17
In veterinary medicine, LUS initially was used in horses to evaluate recurrent airway obstruction, exercise‐induced pulmonary hemorrhage, pulmonary fibrosis, and interstitial pneumonia.20 Recent studies in dogs indicate that LUS may be a useful technique to aid in the diagnosis of cardiogenic PE.21, 22, 23 However, to the best of our knowledge, no studies have described LUS findings in dogs with preclinical CVHD as compared to dogs in CHF.
The aims of our study were to describe LUS features in dogs with different stages of CVHD in accordance with the ACVIM consensus classification system, and to determine the diagnostic accuracy of LUS to detect PE using thoracic radiography as the reference standard.
Materials and Methods
The study protocol and informed consent were reviewed and approved by the Institutional Welfare and Ethics Committee of the University of Pisa (permission number 33472/2016).
Dogs were prospectively recruited from August 2015 to September 2016 at the Department of Veterinary Sciences of the University of Pisa and the Department of Cardiology of the Istituto Veterinario di Novara. Each dog underwent a physical examination, echocardiography, thoracic radiography, and LUS examination.
Inclusion criteria were the presence of a typical heart murmur and an echocardiographic diagnosis of CVHD, characterized by degenerative changes in the mitral valve leaflets, mitral valve prolapse, and the presence of systolic mitral regurgitant flow.24
Collected echocardiographic data were normalized left ventricular internal diameter in diastole (LVIDDn), left atrial‐to‐aortic root ratio (LA/Ao), and flow data including peak velocity of E wave of transmitral flow (Emax) and E wave to A wave ratio of transmitral flow (E/A ratio).
Dogs with CVHD were classified into stages B1, B2, C, and D according to the ACVIM classification scheme.6 Stage B was defined as subclinical heart disease without (B1) or with (B2) evidence of left cardiomegaly, defined as LA/Ao ≥ 1.6,25 LVIDDn > 1.73,26 or both. Dogs were assigned to stage C if they had a history or current clinical signs of CHF in conjunction with past or current evidence of PE on thoracic radiographs. Finally, dogs with past or current evidence of PE on thoracic radiographs that had been treated and relapsed or failed to respond to the initial treatment were classified as stage D.6
Dogs with pleural effusion were excluded from the study. Moreover, dogs with another concomitant heart disease in addition to CVHD were excluded.
Latero‐lateral and orthogonal (ventrodorsal or dorsoventral) radiographic views of the thorax were evaluated. A board‐certified radiologist (E.A) and a radiologist (S.C) reviewed the images independently. All studies were randomly ordered and radiologists were blinded to the animal's history, examination date, initial radiological interpretation and echocardiographic diagnosis, as well as LUS findings. After interpreting the films, the 2 readers’ results were compared. If there was disagreement with regard to the presence or absence of CHF, the two radiologists collectively reassessed the images to arrive at a final definitive diagnosis that satisfied both readers. Radiographic patterns of PE were classified according to location as follows: diffuse (when all the lung fields were involved), perihilar (when only the region surrounding the lung hilum was involved), or focal (when a single area of ≥1 lung lobes was involved).27
Each dog underwent LUS examination on the same day that echocardiography and thoracic radiography were performed. The imaging examinations were performed with no clinically relevant time delay and no additional medical interventions for PE treatment in between. Two sonographers (T.M., E.A.) performed the LUS studies using linear probes1 , 2 with two different ultrasound machines.3 , 4 To avoid lung atelectasis caused by recumbency, dogs were positioned in standing position and manually restrained. Hair was not clipped; alcohol and gel were used as coupling agents. Each hemithorax was examined by sliding the probe from dorsal to ventral, examining all intercostal spaces. Based on a previous study,22 the presence of B‐lines was evaluated as follows: absent (no B‐lines per hemithorax); rare (≤3 B‐lines per hemithorax); numerous (>3 B‐lines per hemithorax); and confluent (multiple B‐lines blended together per hemithorax) (Fig 1). When we detected different LUS findings between the 2 hemithoraces, the most severe finding was assigned.
Figure 1.
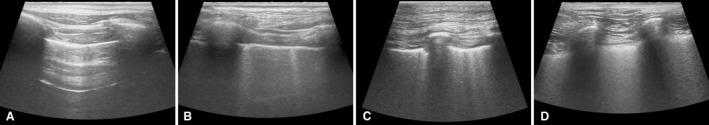
Lung ultrasound images. (A) Absent B‐lines (no B‐lines). (B) Rare B‐lines (≤3 B‐lines). (C) Numerous B‐lines (>3 B‐lines). (D) Confluent B‐lines (multiple B‐lines blended together).
Statistical Analysis
The normality of data distribution was tested using the Shapiro–Wilk test. Descriptive statistics were generated. A value of P < .05 was considered statistically significant.
Differences in continuous data among dogs with different stages of CVHD (B1, B2 and C) were determined by one‐way analysis of variance with subsequent comparisons using Tukey's multiple comparisons test (for normally distributed data) or by the Kruskal–Wallis test, with subsequent comparisons using the Dunn test (for non‐normally distributed data). For categorical variables, a comparison among different stages of CVHD was performed using Fisher's exact tests.
To test the diagnostic accuracy of LUS in the detection of PE, dogs were divided into the following two groups: absent/rare B‐lines versus numerous/confluent B‐lines.8, 13, 22, 23, 28 The sensitivity, specificity, and positive and negative predictive values of LUS to detect PE were calculated using thoracic radiography as the reference standard.
For the statistical analyses, commercial software5 was used.
Results
The study included 63 dogs with a diagnosis of CVHD: 15 dogs in ACVIM stage B1, 18 dogs in ACVIM stage B2, and 30 dogs in ACVIM stage C. A total of 20 of 30 dogs in ACVIM stage C (66.7%) had radiographic signs of PE. In dogs with PE, 14 of 20 (70%) had a focal radiographic pattern of location, 3 of 20 (15%) a diffuse location and 3 of 20 (15%) a perihilar location. Baseline clinical characteristics of the dogs are presented in Table 1. There were no statistically significant differences among groups in terms of sex, age and body weight. Regarding echocardiographic data, dogs in stage C had a significantly higher value of LA/Ao (median, 2.30; range, 1.75–3.68) in comparison with stage B2 (median, 1.80; range, 1.60–2.80) (P < .001) and stage B1 (median, 1.39; range, 1.00–1.50) (P < .001). Left atrial dimension in stage B2 was significantly larger than stage B1 (P < .001). The peak E wave velocity of the diastolic transmitral flow was significantly higher in stage C (median, 1.51 m/s; range, 0.66, 2.30 m/s) in comparison with stage B2 (median, 0.98 m/s; range, 0.40–2.04 m/s) (P < .001) and stage B1 (median, 0.89 m/s; range, 0.66–1.02 m/s) (P < .001). Similarly, the E wave to A wave ratio of the transmitral flow was significantly higher only in stage C (median, 2.04; range, 0.67–4.20) in comparison with stage B2 (median, 1.10; range, 0.70–3.00) (P < .001) and B1 (median, 1.33; range, 0.75–1.65) (P < .001). Lastly, dogs in stage C had a higher value of LVIDDn (median, 2.21; range, 1.63–2.86) in comparison with stage B2 (median, 1.87; range, 1.39–2.34); (P < .001) and stage B1 (median, 1.48; range, 1.25–1.85); (P < .001). The LVIDDn in stage B2 was significantly larger than in stage B1 (P < .001).
Table 1.
Clinical, echocardiographic, and radiographic data of all dogs (n = 63)
| Stage B1 (n = 15) | Stage B2 (n = 18) | Stage C (n = 20) | |
|---|---|---|---|
| Male/Female | 7/8 | 11/7 | 20/10 |
| Age (years) | 11.5 (6.0–15.0) | 11 (6.0–16.0) | 11 (5.0–18.0) |
| BW (kg) | 12 (3.5–40.0) | 10 (4.5–27.0) | 9 (3.7–37.0) |
| LA/Ao | 1.39 (1.00–1.50) | 1.8 (1.60–2.80)a | 2.3 (1.75–3.68)a , b |
| Emax (m/s) | 0.89 (0.66–1.02) | 0.98 (0.40–2.04) | 1.51 (0.66–2.30)a , b |
| E/A ratio | 1.33 (0.75–1.65) | 1.10 (0.70–3.00) | 2.04 (0.67–4.20)a , b |
| LVIDDn | 1.48 (1.25–1.85) | 1.87 (1.39–2.34)a | 2.21 (1.63–2.86)a , b |
| PE (%) | 0 (0%) | 0 (0%) | 20 (66.7%)a , b |
BW, body weight; LA/Ao, left atrium to aortic root ratio; Emax, peak velocity of E wave of the transmitral flow; E/A, E wave to A wave ratio of the transmitral flow; LVIDDn, normalized left ventricular internal diameter in diastole; PE, pulmonary edema.
Data are expressed as the median (range) or number (percentage).
P < .05 as compared to stage B1.
P < .05 as compared to stage B2.
The LUS findings in our sample population are presented in Table 2. Dogs in stage B1 had absent or rare B‐lines in 93.3% of cases (14/15). More specifically, 73.3% (11/15) had absent B‐lines and 20% (3/15) had rare B‐lines. Only 6.7% of dogs in stage B1 (1/15) showed numerous B‐lines. Dogs in stage B2 had absent or rare B‐lines in 88.9% of cases (16/18). Specifically, 66.7% of dogs in stage B2 (12/18) had absent B‐lines and 22.2% (4/18) had rare B‐lines. Only 11.1% of dogs in stage B2 (2/18) showed numerous B‐lines. Similarly, all dogs in stage C (10/10), without radiographic signs of PE, had absent or rare B‐lines. Of the dogs in stage C without radiographic signs of PE, 60% (6/10) had absent B‐lines and 40% (4/10) had rare B‐lines. Dogs in stage C with radiographic signs of PE showed numerous or confluent B‐lines in 90% of cases (18/20), with 55% (11/20) showing numerous B‐lines and 20% (7/20) showing confluent B‐lines, only 10% (2/20) had rare B‐lines.
Table 2.
B‐lines of all dogs (n = 63)
| Stage B1 (n = 15) | Stage B2 (n = 18) | Stage C (noPE) (n = 10) | Stage C (PE) (n = 20) | |
|---|---|---|---|---|
| Absent/rare | 14 (93.3%) | 16 (88.9%) | 10 (100%) | 2 (10%) |
| Numerous/confluent | 1 (6.7%) | 2 (11.1%) | 0 (0%) | 18 (90%) |
noPE, absence of pulmonary edema; PE, pulmonary edema.
Lung ultrasound examination had a 90.0% sensitivity and 93.0% specificity in differentiating dogs with or without PE as assessed by thoracic radiography, with positive predictive value of 85.7% and negative predictive value of 95.2% (Table 3).
Table 3.
Diagnostic accuracy of LUS in the detection of pulmonary edema (n = 63)
| No PE (n = 43) | PE (n = 20) | |
|---|---|---|
| Absent/rare | 40 (93%) | 2 (10%) |
| Numerous/confluent | 3 (7%) | 18 (90%) |
Sensitivity 90.0%; specificity 93.0%; positive predictive value 85.7%; negative predictive value 95.2%.
Discussion
To the authors’ knowledge, ours is the first study describing LUS findings in dogs with different stages of CVHD in accordance with the ACVIM classification scheme. We found that the majority of dogs in stages B1 and B2 had absent or rare B‐lines. Similarly, all dogs in stage C without radiographic signs of PE had absent or rare B‐lines. Conversely, the majority of dogs in stage C with radiographic signs of PE had numerous or confluent B‐lines. These results are in agreement with findings in human patients in whom the number of B‐lines increases with a worsening of the heart failure class,9, 29 and PE is diagnosed when numerous or confluent B‐lines are detected.8, 9, 18 Similarly, previous studies in dogs have shown that cardiogenic PE is associated with numerous or confluent B‐lines on LUS. However, these studies only compared healthy dogs and dogs with radiographic signs of PE.21, 22, 23
In our study, LUS had good diagnostic accuracy in the detection of PE in dogs with CVHD, with sensitivity of 90.0% and specificity of 93.0%. To our knowledge, no previous studies have described the diagnostic accuracy of LUS in the detection of PE in dogs with different stages of CVHD. Our results are similar to findings in people where the sensitivity and specificity of LUS in detecting PE were 83–97% and 83–100%, respectively.8, 16, 28, 30, 31
In our study, a few dogs in stage B1 (6.7%, 1/15) and B2 (11.1%, 2/18) had numerous B‐lines. A possible explanation might be the presence of an underlying pulmonary disease other than cardiogenic PE that was not detected by conventional radiography. B‐lines have been described in people and horses with pulmonary fibrosis, acute respiratory distress syndrome, pulmonary hemorrhages, pneumonia, or lung cancer.13, 18, 20, 32 Another possible explanation for dogs in stages B1 and B2 with numerous B‐lines might be the presence of mild cardiogenic PE, which was not detected by thoracic radiography. Although thoracic radiography is considered the reference standard for the diagnosis of PE in clinical practice, inter‐reader variability may occur.7, 33 Studies in humans indicate that thoracic radiography can be less sensitive than LUS in identifying the presence of cardiogenic PE.30
Our study did not find 100% sensitivity of LUS for the detection of PE, similar to the literature in people.8, 16, 28, 29, 30, 31 Two dogs in stage C with radiographic evidence of PE exhibited only rare B‐lines on LUS in our study. In both cases, the pulmonary infiltrate was only perihilar. It is hypothesized that in patients with only perihilar edema, normally ventilated peripheral lung tissue prevents B‐lines, which would otherwise be visible if pathologic pulmonary parenchyma was present along the thoracic wall, from being seen.
We recognize that our study has some limitations. The number of dogs enrolled was relatively small. However, the sample population was homogeneous. Indeed, only dogs with CVHD were included. It is assumed that a larger number of dogs would have provided similar results. Moreover, intra‐ and interobserver variability during the LUS acquisition was not evaluated. The evaluation of the number of B‐lines might have been partially biased by the operator. However, many studies in people have shown that intra‐ and interobserver variability is clinically acceptable if the procedure is performed by trained operators.34, 35, 36 A linear probe was used in all dogs for the LUS examination. However, currently no consensus exists in the veterinary literature regarding the best type of probe to be used for LUS.21, 22, 23 The results of our study may have differed slightly using another type of probe, but linear high frequency probes are thought to provide the best resolution for scanning superficial structures. Lastly, we used thoracic radiography as the reference method to evaluate the pulmonary parenchyma. Computed tomography is a more sensitive and specific technique than thoracic radiography to diagnose cardiogenic PE.37 However, both in people and in dogs, thoracic radiography is the first line procedure to assess pulmonary congestion, and computed tomography is not a routine technique in patients with heart failure, especially in acute PE.1, 6, 37
Our findings indicate that LUS has good diagnostic accuracy in identifying cardiogenic PE and might be useful in the staging of dogs with CVHD. Lung ultrasound examination is a new, quick, and noninvasive diagnostic tool for the cardiologist, radiologist, or intensive care specialist. It should be considered as complementary to thoracic radiography, and particularly useful when radiographic findings are unclear or in severely dyspneic dogs. In the future, it would be interesting to evaluate the utility of LUS in the chronic management and serial monitoring of dogs with CVHD under treatment.
Acknowledgments
The authors are grateful to Damiano Frangioni for his technical assistance.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work was carried out at the Department of Veterinary Sciences, University of Pisa, and at the Istituto Veterinario di Novara (Italy).
The study was not supported by a grant.
Footnotes
18L7, 10–14 MHz, Toshiba, Monza Brianza
9L‐D, 2.4–10.0 MHz, GE Healthcare, Milano
Aplio, Toshiba, Monza Brianza
Logiq S8, GE Healthcare, Milano
GraphPad Prism 5
References
- 1. Buchanan JW. Chronic valvular disease (endocardiosis) in dogs. Adv Vet Sci Comp Med 1977;21:75–106. [PubMed] [Google Scholar]
- 2. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 3. MacDonald KA, Kittleson MD, Munro C, et al. Brain natriuretic peptide concentration in dogs with heart disease and congestive heart failure. J Vet Intern Med 2003;17:172–177. [DOI] [PubMed] [Google Scholar]
- 4. Häggström J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]
- 5. Boswood A, Häggström J, Gordon SG, et al. Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: The EPIC study‐a randomized clinical trial. J Vet Intern Med 2016;30:1765–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 7. Schober KE, Hart TM, Stern JA, et al. Detection of congestive heart failure in dogs by Doppler echocardiography. J Vet Intern Med 2010;24:1358–1368. [DOI] [PubMed] [Google Scholar]
- 8. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008;134:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miglioranza MH, Gargani L, Sant'Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: A comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013;6:1141–1151. [DOI] [PubMed] [Google Scholar]
- 10. Blanco PA, Cianciulli TF. Pulmonary edema assessed by ultrasound: Impact in cardiology and intensive care practice. Echocardiography 2016;33:778–787. [DOI] [PubMed] [Google Scholar]
- 11. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: The comet‐tail artifact. Intensive Care Med 1998;24:1331–1334. [DOI] [PubMed] [Google Scholar]
- 12. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar‐interstitial syndrome. Am J Emerg Med 2006;24:689–696. [DOI] [PubMed] [Google Scholar]
- 13. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012;38:577–591. [DOI] [PubMed] [Google Scholar]
- 14. Lichtenstein D, Meziere G, Biderman P, et al. The comet‐tail artifact. An ultrasound sign of alveolar‐interstitial syndrome. Am J Respir Crit Care Med 1997;156:1640–1646. [DOI] [PubMed] [Google Scholar]
- 15. Volpicelli G, Caramello V, Cardinale L, et al. Detection of sonographic B‐lines in patients with normal lung or radiographic alveolar consolidation. Med Sci Monit 2008;14:122–128. [PubMed] [Google Scholar]
- 16. Baldi G, Gargani L, Abramo A, et al. Lung water assessment by lung ultrasonography in intensive care: A pilot study. Intensive Care Med 2012;39:74–84. [DOI] [PubMed] [Google Scholar]
- 17. Agricola E, Bove T, Oppizzi M, et al. “Ultrasound comet‐tail images”: A marker of pulmonary edema: A comparative study with wedge pressure and extravascular lung water. Chest 2005;127:1690–1695. [DOI] [PubMed] [Google Scholar]
- 18. Gargani L. Lung ultrasound: A new tool for the cardiologist. Cardiovasc Ultrasound 2011;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Platz E, Lewis EF, Uno H, et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J 2016;37:1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reef VB, Whittier M, Allam LG. Thoracic ultrasonography. Clinical Techniques in Equine Practice 2004;3:284–293. [Google Scholar]
- 21. Louvet A, Bourgeois JM. Lung ring‐down artifact as a sign of pulmonary alveolar‐interstitial disease. Vet Radiol Ultrasound 2008;49:374–377. [DOI] [PubMed] [Google Scholar]
- 22. Lisciandro GR, Fosgate GT, Fulton RM. Frequency and number of ultrasound lung rockets (B‐lines) using a regionally based lung ultrasound examination named vet BLUE (veterinary bedside lung ultrasound exam) in dogs with radiographically normal lung findings. Vet Radiol Ultrasound 2014;55:315–322. [DOI] [PubMed] [Google Scholar]
- 23. Rademacher N, Pariaut R, Pate J, et al. Transthoracic lung ultrasound in normal dogs and dogs with cardiogenic pulmonary edema: A pilot study. Vet Radiol Ultrasound 2014;55:447–452. [DOI] [PubMed] [Google Scholar]
- 24. Chetboul V, Tissier R. Echocardiographic assessment of canine degenerative mitral valve disease. J Vet Cardiol 2012;14:127–148. [DOI] [PubMed] [Google Scholar]
- 25. Rishniw M, Erb HN. Evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 26. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 27. Diana A, Guglielmini C, Pivetta M, et al. Radiographic features of cardiogenic pulmonary edema in dogs with mitral regurgitation: 61 cases (1998–2007). J Am Vet Med Assoc 2009;235:1058–1063. [DOI] [PubMed] [Google Scholar]
- 28. Russell FM, Ehrman RR, Cosby K, et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea: A lung and cardiac ultrasound (LuCUS) protocol. Acad Emerg Med 2015;22:182–191. [DOI] [PubMed] [Google Scholar]
- 29. Frassi F, Gargani L, Tesorio P, et al. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail 2007;13:830–835. [DOI] [PubMed] [Google Scholar]
- 30. Pivetta E, Goffi A, Lupia E, et al. Lung ultrasound‐implemented diagnosis of acute decompensated heart failure in the ED: A SIMEU multicenter study. Chest 2015;148:202–210. [DOI] [PubMed] [Google Scholar]
- 31. Pesenti A, Musch G, Lichtenstein D, et al. Imaging in acute respiratory distress syndrome. Intensive Care Med 2016;42:686–698. [DOI] [PubMed] [Google Scholar]
- 32. ReiBig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease. J Ultrasound Med 2003;22:173–180. [DOI] [PubMed] [Google Scholar]
- 33. Henriksson L, Sundin A, Smedby O, et al. Assessment of congestive heart failure in chest radiographs. Observer performance with two common film‐screen systems. Acta Radiol 1990;31:469–471. [PubMed] [Google Scholar]
- 34. Bedetti G, Gargani L, Corbisiero A, et al. Evaluation of ultrasound lung comets by hand‐held echocardiography. Cardiovasc Ultrasound 2006;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jambrik Z, Monti S, Coppola V, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 2004;15:1265–1270. [DOI] [PubMed] [Google Scholar]
- 36. Shah S, Noble VE, Umulisa I, et al. Development of an ultrasound training curriculum in a limited resource international setting: Successes and challenges of ultrasound training in rural Rwanda. Int J Emerg Med 2008;1:193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cardinale L, Priola AM, Moretti F, et al. Effectiveness of chest radiography, lung ultrasound and thoracic computed tomography in the diagnosis of congestive heart failure. World J Radiol 2014;6:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]


