Abstract
Background
Gastroesophageal reflux (GER) is poorly characterized in anesthetized cats, but can cause aspiration pneumonia, esophagitis, and esophageal stricture formation.
Objective
To determine whether pre‐anesthetic orally administered omeprazole increases gastric and esophageal pH and increases serum gastrin concentrations in anesthetized cats, and to determine the prevalence of GER using combined multichannel impedance and pH monitoring.
Animals
Twenty‐seven healthy cats undergoing elective dental procedures.
Methods
Prospective, double‐masked, placebo‐controlled, randomized clinical trial. Cats were randomized to receive 2 PO doses of omeprazole (1.45–2.20 mg/kg) or an empty gelatin capsule placebo 18–24 hours and 4 hours before anesthetic induction. Blood for measurement of serum gastrin concentration was collected during anesthetic induction. An esophageal pH/impedance catheter was utilized to continuously measure esophageal pH and detect GER throughout anesthesia.
Results
Mean gastric pH in the cats that received omeprazole was 7.2 ± 0.4 (range, 6.6–7.8) and was significantly higher than the pH in cats that received the placebo 2.8 ± 1.0 (range, 1.3–4.1; P < .001). Omeprazole administration was not associated with a significant increase in serum gastrin concentration (P = .616). Nine of 27 cats (33.3%) had ≥1 episode of GER during anesthesia.
Conclusions and Clinical Relevance
Pre‐anesthetic administration of 2 PO doses of omeprazole at a dosage of 1.45–2.20 mg/kg in cats was associated with a significant increase in gastric and esophageal pH within 24 hours, but was not associated with a significant increase in serum gastrin concentration. Prevalence of reflux events in cats during anesthesia was similar to that of dogs during anesthesia.
Keywords: Esophagitis, Feline, Gastric pH, Impedance, Reflux
Abbreviations
- COV
coefficient of variation
- GEJ
gastroesophageal junction
- GER
gastroesophageal reflux
- LES
lower esophageal sphincter
- MII
multichannel intraluminal impedance monitoring
- NANC
nonadrenergic noncholinergic
- PPI
proton pump inhibitor
- TLESRs
transient lower esophageal sphincter relaxations
- VMTH
Veterinary Medical Teaching Hospital
Gastroesophageal reflux (GER) and its associated sequelae are well‐documented causes of morbidity and potential mortality in several species, including people, dogs, and cats.1, 2, 3, 4, 5, 6 The lower esophageal sphincter (LES) is the main physiologic barrier to GER by maintenance of LES tone.1, 7, 8 Maintenance of tone is complex and involves neurologic (vagal, nonadrenergic noncholinergic) and mechanical (diaphragmatic crural pressure) inputs.7, 8 Anesthesia‐related GER was a suspected cause of esophageal stricture in 64–71% of cats after anesthesia,3, 5, 6 but only 2 studies have assessed the incidence of GER in anesthetized cats.9, 10 One study used pH‐metry to determine the incidence of GER in anesthetized adult cats and documented an incidence ranging from 12 to 16%, depending on the type of anesthesia.9 A second study in kittens using a single anesthetic protocol documented GER in 23% of the kittens.10 Relaxation of the LES associated with the administration of injectable and inhaled anesthetic agents represents a primary mechanism for the increased incidence of reflux.11, 12
The diagnosis of GER in cats can be challenging given the intermittent nature of the problem, lack of overt clinical signs, and the need for advanced diagnostic equipment including esophageal pH catheters or pH capsules, endoscopes, and videofluoroscopic units that are not readily available to many veterinarians. Videofluoroscopy also is challenging to complete in nonsedated cats given the temperament of the animals during restraint and the reluctance of cats to readily swallow barium‐soaked food.13 Esophageal pH monitoring was considered the gold standard for documenting GER in people and animals, but mounting evidence has documented that esophageal pH probes underestimate the frequency of reflux events (RE) because of their insensitivity to nonacid reflux and subtle changes in pH that can be associated with many reflux events.14, 15, 16 Combined multichannel intraluminal impedance (MII)/pH‐metry uses impedance technology to determine the type (liquid, gas) and incidence of reflux independent of its acidity.14, 16 Combined MII/pH‐metry also allows for detection of weakly acidic reflux (4.0 < pH < 7.0) and weakly alkaline (pH ≥ 7.0) reflux events that have been associated with esophagitis and respiratory symptoms in people.14, 17, 18
Previous studies evaluating GER in anesthetized cats exclusively have evaluated the incidence of reflux under anesthesia using esophageal pH probes.9, 10 Omeprazole has been evaluated in humans and dogs to decrease GER under anesthesia, but most studies have documented no effect of proton pump inhibitors (PPIs) on frequency of RE. Prolonged exposure of the esophageal mucosa to refluxed acid is an important cause of esophagitis and potential stricture formation, particularly when pH is <4.0 because the proteolytic pH range for the conversion of pepsinogen to pepsin is between 1.5 and 3.5.19 Efforts to neutralize gastric acid by PO administration of PPIs within 24 hours of anesthesia induction and determination of the effects of 2 PO doses of a PPI on serum gastrin concentrations have not been determined in cats to date.20, 21, 22, 23, 24, 25 Long‐term PPI therapy induces moderate hypergastrinemia and enterochromaffin‐like (ECL)‐cell hyperplasia in most patients, and hypergastrinemia has been used as a surrogate marker of gastric acid suppression efficacy.26 Gastrin concentrations therefore may be a comparably accurate measure of pharmacodynamic antisecretory effects vs. measurements of gastric acid secretion.26
We hypothesized that pre‐anesthetic administration of omeprazole in cats undergoing elective periodontal procedures would increase gastric and esophageal pH as well as serum gastrin concentrations. In addition, we hypothesized that the prevalence of GER in cats during anesthesia was significantly lower than that of dogs given the higher prevalence of clinically apparent esophagitis and postanesthesia stricture formation in dogs.
Materials and Methods
Animals
Client‐owned cats admitted to the William R. Pritchard Veterinary Medical Teaching Hospital (VMTH) at the University of California, Davis, for elective dental procedures were recruited for inclusion into this prospective, randomized, masked, placebo‐controlled study. All cats <6 years underwent a comprehensive physical examination, followed by a minimum database consisting of measurement of hematocrit and plasma protein concentration, semiquantitative assessment of blood urea nitrogen by dipstick,1 and urine specific gravity (USG) with a refractometer.2 In addition, a CBC, biochemistry panel, urinalysis, and serum concentration of total T4 were performed on all cats ≥6 years old. Cats with a history of GER, regurgitation, vomiting, esophagitis, and coughing or cats that had received a gastric acid suppressant drug or prokinetic medication within 2 weeks of anesthesia were excluded. Hyperthyroid cats or cats with renal disease (IRIS stage I or higher) also were excluded from the study. All laboratory testing must have been completed within 1 month of the procedure date. A sample size of 10 cats in each group, assuming a standard deviation (SD) of 1.0, a 2‐sided test, and a probability of type 1 error equal to 0.05 had a power of 0.92 to detect a mean difference in pH of 1.5. We chose to enroll 13 cats in each group to account for attrition and achieved the desired level of power. The study protocol was evaluated and approved by the University of California, Davis, School of Veterinary Medicine Animal Care and Use Committee (IACUC), and owners of all cats signed an informed consent form before enrollment of the pet in the study.
Treatment Groups
Each cat was randomized by an Excel software program3 random number generator into 1 of 2 treatment groups to receive either 2 PO doses of omeprazole sodium4 (1.45–2.20 mg/kg) or a placebo of an empty gel cap (size # 4) followed by 10 mL of water administered PO by syringe. Omeprazole capsules (20 mg) containing enteric‐coated omeprazole granules were opened and the contents evenly divided by weight to formulate 10 mg capsules that again were placed into empty gel caps (size # 4). All treatments were administered by 1 of the investigators who was not involved in the interpretation of the pH/impedance results. A first dose of omeprazole or placebo was given PO 18–24 hours before induction, and a second dose was administered PO 4 hours before induction.
Gastrin Assay
Blood was collected into a serum tube (without serum separator gel) from the cephalic or medial saphenous vein at the time of IV catheter placement within 15 minutes of anesthetic induction. Serum samples were stored at −80°C until being batch‐mailed for determination of serum gastrin concentrations at Michigan State University Diagnostic Center for Population and Animal Health. Serum gastrin concentration was determined with a commercially available radioimmunoassay kit according to the manufacturer's protocol.5 The laboratory reported the following percentage cross‐reactivity with related compounds: gastrin 17‐I (100%), gastrin 17‐II (77%), gastrin 34‐I (42%), gastrin 5–17 (54%), cholecystokinin‐PZ (<0.1%), and cholecystokinin‐8 (10.9%). The laboratory‐reported limit of detection was 3 ng/L. For intra‐assay repeatability (10 replicates), the coefficient of variation (COV) for a feline sample with a gastrin concentration of 45 ng/L was 8.6%. For interassay repeatability (10 replicates), the COV for a feline sample with a gastrin concentration of 54 ng/L was 9.2%.
Anesthetic Protocol
All cats were fasted for at least 12 hours before induction. The anesthetic protocol was identical in each cat, consisting of premedication with oxymorphone6 (0.05 mg/kg SC) and atropine7 (0.02 mg/kg SC), followed by induction of anesthesia with propofol8 (4 mg/kg IV) and midazolam9 (0.2 mg/kg IV), titrated to effect to produce a lack of palpebral reflex, ventromedial rotation of the eyes, and jaw muscle relaxation. Anesthesia was maintained in all cats with isoflurane10 (1–3%), titrated to maintain appropriate procedural anesthetic depth. All cats were endotracheally intubated and maintained in dorsal recumbency, and lactated Ringer's solution11 (10 mL/kg/h IV) was administered throughout anesthesia. Esophageal temperature probes and stethoscopes were avoided during anesthetic monitoring to minimize artifact during recording. The anesthetist responsible for administering and monitoring anesthesia was masked to the treatment group assignment.
Measurement of Reflux
Immediately after induction of anesthesia, a 6.4‐French (2.13‐mm) esophageal multi‐use pH/impedance catheter12 was attached to an electrical external reference pad that was placed in the axillary region of the cat. All of the pH/impedance catheters had 7 impedance sensors, each in the form of a 4‐mm cylindrical ring and spaced 2 cm apart, as well as 1 pH sensor located approximately 2 cm from the tip of the catheter (Fig 1). The segment between each pair of sensors, known as the impedance sensor spacing, corresponds to 1 recording impedance channel, thus resulting in 6 corresponding impedance channels along the length of the catheter.
Figure 1.

Photograph of the 6.4‐French (2.1‐mm) esophageal multi‐use pH/impedance catheter showing impedance channels (arrow heads), each 2 cm in length in between the impedance sensors, each in the form of a 4‐mm cylindrical ring, and one pH sensor (arrow heads and arrow). The catheter is attached to the recording device (ZepHr) from which data are uploaded onto a computer using proprietary software.
The pH electrode of the MII‐pH catheter was calibrated within 10 minutes of use in buffer solutions of pH 4.0 and 7.0 according to the manufacturer's instructions. The esophageal catheter was coated with a water‐based lubricant13 and introduced into the left or right naris and passed through the ventral nasal meatus into the oropharynx where it was reflected rostrally with the aid of a spay hook. Any residual lubricant was removed from the catheter with a saline‐moistened gauze, and the catheter then was guided aborad into the esophagus by use of a loop snare14 passed through the biopsy channel of a video endoscope.15 Catheter placement was performed in all cats by 1 of 3 investigators (SLM, RSG, or ADM) skilled in endoscope handling to ensure consistency in the positioning of the catheter. The catheter was advanced into the greater curvature region of the stomach in 32 cats to record gastric pH for 2 minutes before the catheter was retracted into the distal esophagus so that the pH sensor on the catheter was positioned 6 cm proximal to the gastroesophageal junction (GEJ) in all cats with all of the catheter's impedance rings and channels located within the esophagus. Deionized water was used to rinse any residual gastric acid from the pH catheter, and air introduced into the stomach and esophagus during catheter placement was carefully suctioned. The catheter then was secured in place using butterfly tape wrapped around the catheter and secured to the skin with skin staples16 lateral to the naris and ventrolateral to the ipsilateral zygomatic arch. The catheter was attached to a recording device17 from which data were uploaded to a computer by proprietary software from the manufacturer.18 The reasons for placing the catheter transnasally (in contrast to transorally) were 2‐fold: (1) to minimize interference (movement and displacement) of the catheter during the dental procedure, and (2) to avoid interference with the dentist's procedure and field of vision in the oral cavity. The oropharynx was suctioned throughout the procedure to minimize fluid entering the hypopharynx and esophagus and contaminating the distally located pH sensor or impedance channels. Esophageal pH and impedance were recorded throughout the dentistry procedure until the catheter was removed immediately before extubation of the cat. Data analysis was performed by proprietary software from the manufacturer19 by 1 of the investigators (JO) who was masked to the treatment group assignment.
Defining Reflux by Impedance
A reflux episode was defined as a 50% decrement in ohms seen in 2 consecutive impedance channels in the distal esophagus for >2 seconds from the pre‐episodic esophageal baseline recording. Impedance (Z) technology relies upon the principle of resistance to the passage of flow of an electrical current and is inversely related to conductivity. Impedance is influenced by the physical characteristics of intraluminal substrates, and gastric refluxate has high electrical conductivity, or low impedance, whereas intraluminal air has a low conductivity, or high impedance. Interpretation of the waveform generated by the computer can be used to determine whether the refluxate originated orally or aborally. In addition, the demarcated concentric impedance rings and impedance channels located along the length of the catheter allow the investigator to determine the distance the refluxate travels up the esophagus by assessing the length of the waveform generated (Fig 2).
Figure 2.
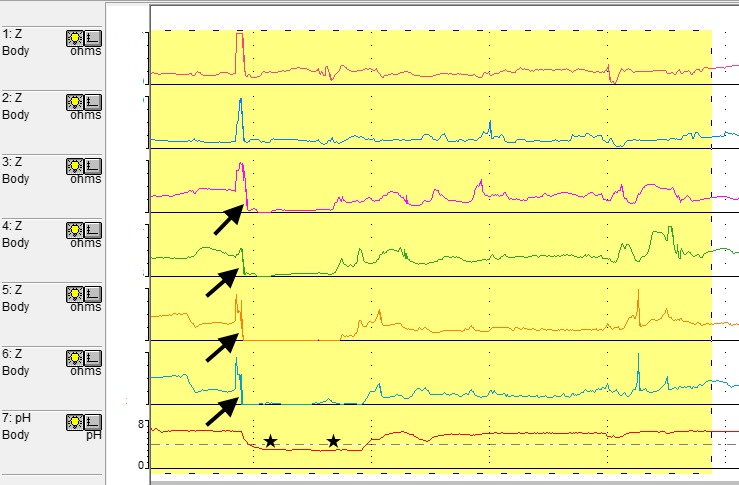
Waveform depicting a strongly acidic reflux event (pH < 4.0 designated by black stars) in an 8.3‐year‐old male‐neutered domestic longhaired cat in the placebo group. The numbered Z channels on the y‐axis represent impedance channels spaced throughout the esophagus with Z channel 1 being the most proximal in the esophagus and Z channel 6 representing the most distal impedance channel. Bolus presence is identified by a drop in impedance by at least 50% of the pre‐episode impedance highlighted by the black arrows on Z channels 3–6. Direction of bolus movement in the esophagus is determined by the direction of the impedance change with reflux moving distal to proximal and swallow moving proximal to distal. The height of reflux is determined by the position of the most proximal channel showing a drop in impedance (Z channel # 3). Although channel # 7 is positioned on the tracing below the impedance channels, the position of the pH sensor in the esophagus is at the same level as the most distal impedance channel.
The pH of the refluxate was classified as strongly acidic (pH < 4.0), weakly acidic (4.0 < pH < 7.0), or weakly alkaline (pH ≥ 7.0). The mean acid clearance time was defined as the duration of each acid RE, beginning at the moment the pH decreased to <4.0 and ending when the pH increased to ≥4.1. All cats were monitored during the dentistry procedure for regurgitation or reflux events by the anesthesiologist, and all events were recorded in the anesthesia record of the animal.
Statistical Analysis
All data were coded and recorded into SPSS 22.0 for the Macintosh computer.20 Differences between group means were assessed by the independent‐samples t‐test. All results were confirmed with the nonparametric Mann‐Whitney U‐test. Statistical significance was ascertained at a probability of type I error (α) of 0.05. A Bonferroni correction was utilized to adjust for multiple comparisons (1 primary and 3 secondary endpoints).
Results
Animals
Thirty‐six cats were evaluated for enrollment in the study, and 9 cats ultimately were excluded: 4 cats were excluded because of small patient size (<4.0 kg) precluding transnasal passage of the esophageal catheter. Three additional cats were excluded because of failure of pH/impedance catheter calibration or failure of pH/impedance data to be recorded. Two cats were excluded postprocedure because of histories of chronic vomiting (1) and chronic kidney disease (1) that were not apparent at the time of enrollment. Of the 27 cats that completed the study, 14 were assigned to the placebo group and 13 were assigned to the omeprazole group. Drying of the pH sensor on the catheter precluded accurate determination of gastric and esophageal pH in 4 of the 27 cats, but this did not affect impedance function, which allowed determination of RE in all cats. Seventeen cats had a CBC, biochemistry panel, urinalysis, and serum T4 concentration performed. Of the remaining 10 cats (all <6 years of age), 8 did not have serum T4 concentration measured, 4 had a hematocrit/plasma protein performed instead of a CBC, and 2 had semiquantitative assessment of blood urea nitrogen by dipstick and measurement of urine specific gravity (USG) instead of a biochemistry panel and complete urinalysis.
The age of the cats ranged from 1.7 to 16.9 years (mean ± SD, 8.6 ± 3.8 years). Body weight ranged from 4.5 to 7.7 kg (mean ± SD, 5.5 ± 0.8 kg). Castrated male cats (18 of 27; 67%) represented the largest sex grouping; the remaining 9 cats were spayed females. No significant differences in age (P = .35), body weight (P = .43), or sex distribution (P = .17) were identified between the treatment groups. Domestic shorthaired cats represented the most common breed (n = 19/27; 70%), with domestic longhaired cats (n = 4/27; 15%), Maine Coon (n = 2/27; 7%), Himalayan (n = 1/27; 4%), and Burmese (n = 1/27; 4%) breeds also represented. The dosage of omeprazole administered to the cats assigned to the omeprazole group ranged from 1.45 to 2.20 mg/kg (mean ± SD, 1.85 ± 0.23 mg/kg). No diarrhea was noted by owners of any cat in the omeprazole group after administration of the drug. The dentistry procedure time ranged from 1.4 to 5.1 hours (mean ± SD, 2.8 ± 0.9 hours), and no significant difference in procedure time was identified between treatment groups (P = .65). All cats underwent periodontal treatment under anesthesia, and 17 of 27 cats (63%) also required dental extractions. There was no difference in the procedures performed between groups (P = .88). No morbidity or death was associated with anesthesia or the study procedures. No visual evidence of reflux or regurgitation was recorded in any of the enrolled cats during the dentistry procedure.
Gastrin
Serum gastrin concentrations were measured in all 27 cats enrolled in the study. Gastrin concentrations in the placebo group (34.3 ± 10.8 ng/L; range, 22–64 ng/L) and omeprazole group (45.7 mean ± 37.2 ng/L; range, 16–135 ng/L) were not significantly different (P = .62).
Gastric pH
Gastric pH was recorded in all 27 cats, but pH readings were invalid for 1 cat in the placebo group and for 3 cats in the omeprazole group because of drying of the pH sensor. The drying of the pH sensor did not affect the functioning of the impedance channels that allowed the continued determination of RE throughout the procedure in these cats. Gastric pH readings were valid in 23 of 27 cats, representing 13 cats in the placebo group and 10 in the omeprazole group. Mean gastric pH in the cats that received omeprazole was 7.2 ± 0.4 (range, 6.6–7.8) and was significantly higher than the pH in cats that received the placebo 2.8 ± 1.0 (range, 1.3–4.1; P < .001; Fig 3).
Figure 3.
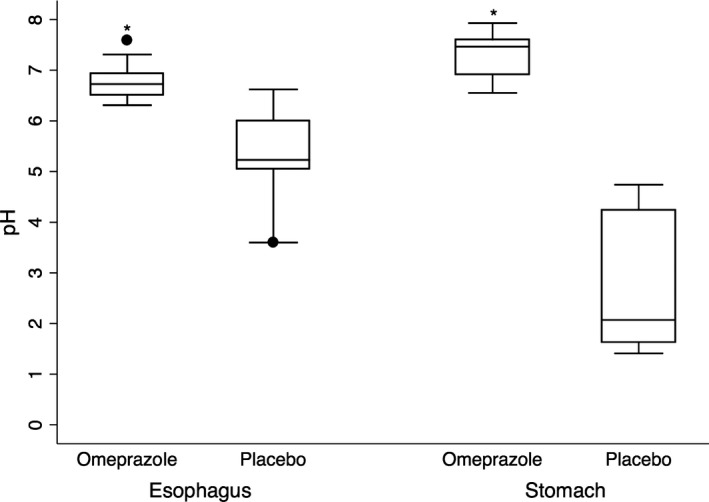
Distribution of gastric and esophageal pH in 23 cats undergoing elective dental procedures after oral administration of omeprazole (n = 10 cats) administered twice at 1.45–2.2 mg/kg 18–24 hours before induction and 4 hrs before induction, or placebo (empty gel cap) (n = 13 cats). The horizontal lines of the box represent, from bottom to top, the 25th, 50th, and 75th percentiles of pH values. Whiskers represent the maximum and minimum pH values, except for outlying observations (solid circles). The symbol “*” denotes the mean esophageal and gastric pH in the cats that received omeprazole was significantly higher than the pH in cats that received the placebo (P < .001)
Esophageal pH
Esophageal pH was measured in the same 23 cats as gastric pH, and results of esophageal pH testing were not valid in the other 4 cats. Mean esophageal pH for the omeprazole group was 6.8 ± 0.4 (range, 6.3–7.6) and was significantly higher (P < .001) than mean pH for the placebo group 5.3 ± 0.9 (range, 3.8–6.6). An esophageal pH < 4.0 was documented in 3 of 13 cats in the placebo group and in none of the cats in the omeprazole group. In these 3 cats, the mean time of esophageal pH <4.0 was 106.5 ± 107.2 minutes (range, 7.1–220.0 minutes), or 48.8% of total procedure time. The mean percentage of total procedural time that the esophageal pH was <4.0 was 14.2% for the entire placebo group. Mean percentage of procedural time that the esophageal pH was <4.0 for the omeprazole group could not be calculated because a pH <4.0 was not recorded.
Gastroesophageal Reflux Events
Nine of 27 cats (33.3%) had at least 1 episode of GER during anesthesia, representing 7 of 14 cats (50.0%) in the placebo group, and 2 of 13 cats (15.4%) in the omeprazole group. In total, 14 individual REs were documented in the 9 cats (Table S1). Of the 9 cats in which reflux was identified, 6 cats each had a single reflux event (5 placebo, 1 omeprazole), a single cat had 2 reflux events (placebo), and 2 cats each had 3 separate reflux events (1 placebo, 1 omeprazole; Table S1).
The height of the refluxate in the esophagus was determined by impedance data for all 14 REs in the 9 cats that experienced reflux (Table S1). No cat was noted to have visual evidence of reflux or regurgitation during or immediately after anesthesia. The mean ± SD number of RE was 0.71 ± 0.94 for the placebo group (n = 14) and 0.31 ± 0.86 for the omeprazole group (n = 13). The administration of omeprazole did not significantly decrease the number of RE in comparison with the placebo group (P = .057). The odds ratio of the placebo group having at least 1 reflux event was 2.75 times the likelihood of a reflux event in the omeprazole group (95% confidence interval [CI] = 0.77–9.86; P = .069).
Mean Acid Clearance Time
The mean acid clearance time was calculated for cats in the placebo group (106.5 ± 107.2 minutes; range, 7.1–220 minutes). Cats in the omeprazole group had no strongly acidic reflux documented, precluding calculation of mean acid clearance time.
Discussion
Gastroesophageal reflux (GER) during anesthesia is a common and well‐documented phenomenon in people and dogs and has been associated with esophagitis, esophageal stricture formation, and aspiration pneumonia.1, 3 In contrast, GER has been documented to occur less frequently in endotracheal‐intubated cats,1, 2, 3, 4, 5, 13 and the refluxate reached the pharynx in <7% of cats compared to nearly 25% of dogs.9, 10, 27 Several pre‐anesthetic and anesthetic agents decrease LES tone and possibly increase the risk of GER in cats.11, 12 These include commonly used drugs such as atropine, acepromazine, propofol, ketamine, and xylazine. Atropine and acepromazine administration to cats was shown to decrease LES pressure to 13.2% of baseline,11 and ketamine administration was shown to decrease LES pressure considerably less compared to administration of propofol, thiopentone, and a combination of xylazine, ketamine, and atropine in 40 cats undergoing elective castration or ovariohysterectomy procedures.12 Although not investigated in cats, inhalant anesthetics also have been shown to decrease LES tone in other species.27, 28
Transient lower esophageal sphincter relaxations (TLESRs) have been found to be the main mechanism for all types of RE in humans and provide an alternate mechanism for GER in cats.29, 30 These TLESRs are vasovagal reflex‐mediated, prolonged relaxations of the LES that occur independent of swallowing. They occur as a normal physiologic response to gastric distention, but not gastric pH, and allow venting of gastric gas in humans, dogs, and cats.29, 30, 31, 32 These TLESRs have been documented to occur in cats under the effects of dissociative anesthetics, making them possibly complicit in RE under anesthesia.30, 31
We found a relatively high incidence of GER in 33% of the anesthetized cats overall and in 50% of cats that received placebo. These findings are much higher than the 12–23% reported previously in intubated anesthetized cats and likely reflect the use of combined impedance/pH technology that allowed for detection of weak or nonacid reflux events as well as strongly acidic reflux events.9, 10 Six of 9 reflux events were weak acid reflux events that could have been missed using the pH monitoring devices previously employed in GER studies in cats. Other factors contributing to these divergent results include different patient populations and different anesthetic protocols.9 Despite the high incidence of reflux events in this study, no cats were observed to have any episodes of regurgitation during the anesthetic procedure or upon recovery, supporting previous studies that showed poor sensitivity for visual detection of reflux.9, 10 Despite the relatively high incidence of GER detected in our cats, none had an abdominal surgical procedure linked to a higher incidence of reflux.33
With the aid of computer analysis, the impedance waveform allows the type (gas vs. liquid), direction (orad with gastric reflux, aborad with swallowing), and classification of the refluxate as strongly acidic (pH < 4), weakly acidic (4 ≤ pH < 7), or weakly alkaline (pH ≥ 7).34 Our study utilized dual MII/pH‐metry for the detection of acid and nonacid GER in cats undergoing general anesthesia. Historically, all previously published veterinary studies assessing GER in dogs and cats utilized pH probes for detection of reflux, with the exception of 1 study in dogs that utilized dual MII/pH‐metry.23 Monitoring of pH without concurrent impedance monitoring technology allows for weakly acidic or weakly alkaline RE, which are characterized by subtle changes in pH, potentially to be missed, resulting in an underestimation of the frequency of reflux.15, 16 If traditional pH‐metry without impedance technology had been utilized in our study, only 3 strongly acidic REs would have been documented out of a total of 14 reflux events.
The PO administration of omeprazole was associated with a significant increase in both gastric and esophageal pH, and none of the 10 cats in the omeprazole group that had continuous gastric and esophageal pH measurements throughout their procedures had a gastric or esophageal pH < 4.0 at any measured time point. This marked effect was noted despite administration of 2 PO doses of the PPI within only 24 hours of anesthesia. In the placebo group, 3 cats had periods of time during which esophageal pH was <4.0, with 2 cats having pH <4.0 for over 90 minutes (92.3 and 220.0 minutes, respectively). In vivo studies in several domestic species, including cats, have shown that acute esophagitis can be induced by bathing the esophagus in a combination of HCl and pepsin, whereas exposure to HCl alone for protracted periods (up to 1 hour) does not result in macroscopic or microscopic changes consistent with acute esophagitis.35, 36, 37 Despite the established risk of pepsin, other enzymes (e.g, trypsin) and bile salts can cause esophagitis.18, 19, 38, 39 These compounds often have maximal proteolytic or solubilizing activities at a pH that can be found in the esophagus after weakly or nonacid RE. This further highlights the importance of characterizing all types of RE, not just strongly acidic RE. Anesthesia increases the risk of esophagitis and stricture formation in part because clearance of esophageal reflux does not occur under anesthesia.
Omeprazole was administered at a slightly higher dosage than has been used in previous studies of cats,40, 41 although the dosage is consistent with studies in dogs showing the efficacy and safety of a higher dosage of omeprazole.42 Twice‐daily dosing of omeprazole in cats recently was shown to cause significant acid suppression in contrast to once‐daily administration of the drug.40
Administration of omeprazole did not significantly decrease the number of RE in comparison with the placebo group (P = .057), consistent with previous impedance/pH‐metry studies in humans and dogs that failed to find a decrease in the incidence of reflux with pre‐anesthetic administration of omeprazole or esomeprazole (the active S‐enantiomer of omeprazole) compared to controls.23, 25 A single study in dogs found that pre‐anesthetic administration of omeprazole decreased the incidence of reflux, but that study was hampered by the use of pH monitoring only to detect reflux events.22 The mechanism responsible for decreased reflux in the omeprazole group is not definitively known, but omeprazole and esomeprazole have been shown to decrease gastric volume in both people and cats and could have decreased the number of TLERs.24, 43
Based on the results of our study showing a relatively high prevalence of reflux events in cats during anesthesia and a significant effect of omeprazole on gastric and esophageal pH, future studies should determine the clinical impact of GER in anesthetized cats and whether pre‐anesthetic administration of PPIs is warranted. Modification of gastric and esophageal pH by omeprazole might decrease the risk of esophagitis and esophageal stricture formation based on experimental studies performed in rabbits and cats.19, 35, 37
The lack of an increase in serum gastrin concentrations after 2 PO doses of omeprazole to the cats in our study was unexpected in light of the findings of increases in serum gastrin concentrations within 24 hours of PPI administration in dogs and humans.44, 45 Serum gastrin measurements in healthy controls in a previous study showed a seemingly wide range of concentrations (17–94 pg/mL) in cats and dogs, with many dogs having serum gastrin concentrations within the normal reference interval 24 hours after PPI administration.45, 46 It took 4–5 days for serum gastrin concentration to peak in dogs, although gastrin concentrations did increase to a level of significance 24 hours after initiation of omeprazole therapy.45 A recent study evaluating the long‐term effects (8 weeks) of twice‐daily omeprazole orally administered to 6 healthy adult cats showed a significant increase in serum gastrin concentrations at 4 weeks and 8 weeks after initiation of omeprazole administration compared to placebo, but serum gastrin concentrations were not determined within the first 24 hours of omeprazole administration.47 It is plausible that the relatively brief period after administration of omeprazole and collection of serum for gastrin determination provided insufficient time for removal of negative‐feedback inhibition of gastrin secretion as has been documented in humans.48 The gastrin assay we used has been validated for cats and has been used in other studies and is not suspected to be a source of error.45 Further studies with larger numbers of animals given omeprazole for a longer duration are indicated to assess the effects of PPIs on serum gastrin concentrations in cats.
Our study had several limitations. The relatively small study numbers of cats in both groups increased the probability of a type II error. A further limitation of the study was the loss of pH data from several cats, including both of the cats that refluxed in the omeprazole group. Finally, the lack of a comprehensive monitoring system after discharge for the cats that refluxed precluded our ability to critically evaluate the impact of these reflux events in the affected cats. Future studies should include postprocedural monitoring for episodes of difficulty swallowing, salivation, regurgitation, odynophagia, and coughing.
In summary, we showed that gastric pH and esophageal pH were significantly increased within 24 hours in cats that were given 2 PO doses of omeprazole 18–24 and 4 hours before anesthesia, respectively, compared to cats that received a placebo, but a commensurate increase in serum gastrin concentrations was not observed. A relatively high incidence of GER was detected in 33% of the study cats under anesthesia, with 50% of the cats in the placebo group exhibiting ≥1 reflux event. The odds ratio of the placebo group having at least 1 reflux event was 2.75 times the likelihood of a reflux event in the omeprazole group (95% CI = 0.77–9.86; P = .069). Additional studies examining the effects of orally administered omeprazole on the incidence and clinical consequences of reflux in a larger cohort of cats are warranted.
Supporting information
Table S1. Table illustrating the mean esophageal and gastric pH, and the frequency, type, and height of the reflux events in 9 of 27 apparently healthy cats that had ≥ 1 reflux event during anesthesia for an elective dental procedure.
Acknowledgments
The authors thank Sandhill Scientific Inc. for providing the pH/impedance catheters utilized in this study.
Sources of Funding: This study was supported in part by a grant from the Center for Companion Animal Health (CCAH) at the University of California, Davis, School of Veterinary Medicine.
Conflict of Interest Declaration: Sandhill Scientific Inc. employed Jean Osborn at the time of the study. She has advanced expertise in interpreting esophageal pH/impedance recordings and interpreted all recordings in a masked fashion.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was conducted at the William R. Pritchard Veterinary Medical Teaching Hospital (VMTH), University of California, Davis.
This study was not presented as an abstract or poster at any meeting.
Footnotes
Azostix® Reagent Strips, Siemens Healthcare Diagnostics Inc., Tarrytown, NY
Reichert® Vet 360 Refractometer, Reichert Analytical Instruments Inc., Depew, NY
Microsoft® Excel® for Mac 2011, Version 14.4.8
Omeprazole delayed‐release capsules USP, Apotex Inc., Toronto, ON, Canada
Gastrin [125I] Radioimmunoassay Kit, MP Biomedicals, Diagnostics Division, Orangeburg, NY
Oxymorphone hydrochloride injection (1 mg/mL), Endo Pharmaceuticals Inc., Malvern, PA
Atropine, Baxter Healthcare Corporation, Deerfield, IL
Propofol, Diprivan, Abbott Laboratories, North Chicago, IL
Midazolam injection USP, West‐Ward Pharmaceuticals, Eatontown, NJ
Isoflurane USP, Piramal Critical Care, Inc., Bethlehem, PA
Lactated Ringer's Solution, Baxter Healthcare Corporation, Deerfield, IL
Esophageal impedance/pH catheters, model #ZPN‐BS‐46E, Sandhill Scientific, Inc., Highlands Ranch, CO
PDI lubricating jelly II, Orangeburg, NY
Vet Oval Loop “Grabber” snare, 2.5 cm loop, Endoscopy Support Services Inc.,® Brewster, NY
Olympus GIF‐P140 gastroscope, Olympus America Inc., Center Valley, PA
Vet One® surgical skin staples, MWI, Boise, ID
ZepHr® Impedance/pH reflux recorder, Sandhill Scientific, Inc., Highlands Ranch, CO
Sandhill Patient Import/Export Utility software version 5.3.0, Sandhill Scientific, Inc., Highlands Ranch, CO
Sandhill BioVIEW Analysis software version 5.5.4.1 and Sandhill pH Analysis software version 4.0.1, Sandhill Scientific, Inc., Highlands Ranch, CO
IBM SPSS version 22.0 software for the Macintosh, IBM Corporation, Armonk, NY
References
- 1. Cotton BR, Smith G. The lower oesophageal sphincter and anaesthesia. Br J Anaesth 1984;56:37–46. [DOI] [PubMed] [Google Scholar]
- 2. Pearson H, Darke PG, Gibbs C, et al. Reflux oesophagitis and stricture formation after anaesthesia: A review of seven cases in dogs and cats. J Small Anim Pract 1978;19:507–519. [DOI] [PubMed] [Google Scholar]
- 3. Adamama‐Moraitou K, Rallis T, Prassinos N, Galatos AD. Benign esophageal stricture in the dog and cat: A retrospective study of 20 cases. Can J Vet Res 2002;66:55–59. [PMC free article] [PubMed] [Google Scholar]
- 4. Adami C, Di Palma S, Gendron K, Sigrist N. Severe esophageal injuries occurring after general anesthesia in two cats: Case report and literature review. J Am Anim Hosp Assoc 2011;47:436–442. [DOI] [PubMed] [Google Scholar]
- 5. Harai BH, Johnson SE, Sherding RG. Endoscopically guided balloon dilatation of benign esophageal strictures in 6 cats and 7 dogs. J Vet Intern Med 1995;9:332–335. [DOI] [PubMed] [Google Scholar]
- 6. Leib MS, Dinnel H, Ward DL, et al. Endoscopic balloon dilation of benign esophageal strictures in dogs and cats. J Vet Intern Med 2001;15:547–552. [DOI] [PubMed] [Google Scholar]
- 7. Farré R, Sifrim D. Regulation of basal tone, relaxation and contraction of the lower oesophageal sphincter. Relevance to drug discovery for oesophageal disorders. Br J Pharmacol 2008;153:858–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kortezova N, Mizhorkova Z, Millusheva E, et al. Non‐adrenergic non‐cholinergic neuron stimulation in the cat lower esophageal sphincter. Eur J Pharmacol 1996;304:109–115. [DOI] [PubMed] [Google Scholar]
- 9. Galatos AD, Savas I, Prassinos NN, Raptopoulos D. Gastro‐oesophageal reflux during thiopentone or propofol anaesthesia in the cat. J Vet Med A Physiol Pathol Clin Med 2001;48:287–294. [DOI] [PubMed] [Google Scholar]
- 10. Sideri AI, Galatos AD, Kazakos GM, Gouletsou PG. Gastro‐oesophageal reflux during anaesthesia in the kitten: Comparison between use of a laryngeal mask airway or an endotracheal tube. Vet Anaesth Analg 2009;36:547–554. [DOI] [PubMed] [Google Scholar]
- 11. Hashim MA, Waterman AE. Effects of acepromazine, pethidine and atropine premedication on lower oesophageal sphincter pressure and barrier pressure in anaesthetised cats. Vet Rec 1993;133:158–160. [DOI] [PubMed] [Google Scholar]
- 12. Hashim MA, Waterman AE. Effects of thiopentone, propofol, alphaxalone‐alphadolone, ketamine and xylazine‐ketamine on lower oesophageal sphincter pressure and barrier pressure in cats. Vet Rec 1991;129:137–139. [DOI] [PubMed] [Google Scholar]
- 13. Levine JS, Pollard RE, Marks SL. Contrast videofluoroscopic assessment of dysphagic cats. Vet Radiol Ultrasound 2014;55:465–471. [DOI] [PubMed] [Google Scholar]
- 14. Zentilin P, Dulbecco P, Savarino E, et al. Combined multichannel intraluminal impedance and pH‐metry: A novel technique to improve detection of gastro‐oesophageal reflux literature review. Dig Liver Dis 2004;36:565–569. [DOI] [PubMed] [Google Scholar]
- 15. Murphy DW, Faust KB, Chiantella VM, Castell DO. Assessment of accuracy of intraesophageal pH probe in a dog model. Dig Dis Sci 1989;34:1079–1084. [DOI] [PubMed] [Google Scholar]
- 16. Hila A, Agrawal A, Castell DO. Combined multichannel intraluminal impedance and pH esophageal testing compared to pH alone for diagnosing both acid and weakly acidic gastroesophageal reflux. Clin Gastroenterol Hepatol 2007;5:172–177. [DOI] [PubMed] [Google Scholar]
- 17. Woodland P, Sifrim D. Esophageal mucosal integrity in nonerosive reflux disease. J Clin Gastroenterol 2014;48:6–12. [DOI] [PubMed] [Google Scholar]
- 18. Moffat RC, Berkas EM. Bile esophagitis. Arch Surg 1965;91:963–966. [DOI] [PubMed] [Google Scholar]
- 19. Hirschowitz BI. Pepsin and the esophagus. Yale J Biol Med 1999;72:133–143. [PMC free article] [PubMed] [Google Scholar]
- 20. Guilloteau P, Le Meuth‐Metzinger V, Morisset J, Zabielski R. Gastrin, cholecystokinin and gastrointestinal tract functions in mammals. Nutr Res Rev 2006;19:254–283. [DOI] [PubMed] [Google Scholar]
- 21. Goldstein RE, Marks SL, Kass PH, Cowgill LD. Gastrin concentrations in plasma of cats with chronic renal failure. J Am Vet Med Assoc 1998;213:826–828. [PubMed] [Google Scholar]
- 22. Panti A, Bennett RC, Corletto F, et al. The effect of omeprazole on oesophageal pH in dogs during anaesthesia. J Small Anim Pract 2009;50:540–544. [DOI] [PubMed] [Google Scholar]
- 23. Zacuto AC, Marks SL, Osborn J, et al. The influence of esomeprazole and cisapride on gastroesophageal reflux during anesthesia in dogs. J Vet Intern Med 2012;26:518–525. [DOI] [PubMed] [Google Scholar]
- 24. Babaei A, Bhargava V, Aalam S, et al. Effect of proton pump inhibition on the gastric volume: Assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2009;29:863–870. [DOI] [PubMed] [Google Scholar]
- 25. Jeske HC, Borovicka J, von Goedecke A, et al. Preoperative administration of esomeprazole has no influence on frequency of refluxes. J Clin Anesth 2008;20:191–195. [DOI] [PubMed] [Google Scholar]
- 26. Robinson M. Review article: Current perspectives on hypergastrinemia and enterochromaffin‐like‐cell hyperplasia. Aliment Pharmacol Ther 1999;13(Suppl. 5):5–10. [DOI] [PubMed] [Google Scholar]
- 27. Wilson DV, Boruta DT, Evans AT. Influence of halothane, isoflurane, and sevoflurane on gastroesophageal reflux during anesthesia in dogs. Am J Vet Res 2006;67:1821–18250. [DOI] [PubMed] [Google Scholar]
- 28. Chassard D, Tournadre JP, Berrada KR, et al. Effect of halothane, isoflurane and desflurane on lower oesophageal sphincter tone. Br J Anaesth 1996;77:781–783. [DOI] [PubMed] [Google Scholar]
- 29. Iwakiri K, Hayashi Y, Kotoyori M, et al. Transient lower esophageal sphincter relaxations (TLESRs) are the major mechanism of gastroesophageal reflux but are not the cause of reflux disease. Dig Dis Sci 2005;50:1072–1077. [DOI] [PubMed] [Google Scholar]
- 30. Kessing BF, Conchillo JM, Bredenoord AJ, et al. Review article: The clinical relevance of transient lower oesophageal sphincter relaxations in gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 2011;33:650–661. [DOI] [PubMed] [Google Scholar]
- 31. Wang C, Zhou DF, Shuai XW, et al. Effects and mechanisms of electroacupuncture at PC6 on frequency of transient lower esophageal sphincter relaxation in cats. World J Gastroenterol 2007;13:4873–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneider JH, Küper MA, Königsrainer A, Brücher BL. Transient lower esophageal sphincter relaxation and esophageal motor response. J Surg Res 2010;159:714–719. [DOI] [PubMed] [Google Scholar]
- 33. Galatos AD, Raptopoulos D. Gastro‐oesophageal reflux during anaesthesia in the dog: The effect of age, positioning and type of surgical procedure. Vet Rec 1995;137:513–516. [DOI] [PubMed] [Google Scholar]
- 34. Skopnik H, Silny J, Heiber O, et al. Gastroesophageal reflux in infants: Evaluation of a new intraluminal impedance technique. J Pediatr Gastroenterol Nutr 1996;23:591–598. [DOI] [PubMed] [Google Scholar]
- 35. Lanas A, Royo Y, Ortego J, et al. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterol 1999;116:97–107. [DOI] [PubMed] [Google Scholar]
- 36. Kiriluk LB, Merendino KA. The comparative sensitivity of the mucosa of the various segments of the alimentary tract in the dog to acid‐peptic action. Surgery 1954;35:547–556. [PubMed] [Google Scholar]
- 37. Dodds WJ, Goldberg HI, Montgomery C, et al. Sequential gross, microscopic and roentgenographic features of acute feline esophagitis. Invest Radiol 1970;5:209–219. [DOI] [PubMed] [Google Scholar]
- 38. Johnson LF, Harmon JW. Experimental esophagitis in a rabbit model. Clinical relevance. J Clin Gastroenterol 1986;8(Suppl 1):26–44. [DOI] [PubMed] [Google Scholar]
- 39. Helm JF. Esophageal acid clearance. J Clin Gastroenterol 1986;8(Suppl):5–11. [DOI] [PubMed] [Google Scholar]
- 40. Šutalo S, Ruetten M, Hartnack S, et al. The Effect of orally administered ranitidine and once‐daily or twice‐daily orally administered omeprazole on intragastric pH in cats. J Vet Intern Med 2015;29:840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parkinson S, Tolbert K, Messenger K, et al. Evaluation of the effect of orally administered acid suppressants on intragastric pH in cats. J Vet Intern Med 2015;29:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med 2011;25:47–54. [DOI] [PubMed] [Google Scholar]
- 43. Coruzzi G, Bertaccini G. Antisecretory activity of omeprazole in the conscious gastric fistula cat: Comparison with famotidine. Pharmacol Res 1989;21:499–506. [DOI] [PubMed] [Google Scholar]
- 44. Festen HP, Tuynman HA, Défize J, et al. Effect of single and repeated doses of oral omeprazole on gastric acid and pepsin secretion and fasting serum gastrin and serum pepsinogen I levels. Dig Dis Sci 1986;31:561–566. [DOI] [PubMed] [Google Scholar]
- 45. Parente NL, Bari Olivier N, Refsal KR, Johnson CA. Serum concentrations of gastrin after famotidine and omeprazole administration to dogs. J Vet Intern Med 2014;28:1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McLeland SM, Lunn KF, Duncan CG, et al. Relationship among serum creatinine, serum gastrin, calcium‐phosphorus product, and uremic gastropathy in cats with chronic kidney disease. J Vet Intern Med 2014;28:827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gould E, Clements C, Reed A, et al. A prospective, placebo‐controlled pilot evaluation of the effect of omeprazole on serum calcium, magnesium, cobalamin, gastrin concentrations, and bone in cats. J Vet Intern Med 2016;30:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crobach LF, Jansen JB, Lamers CB. Effect of intermittent weekend therapy with omeprazole on basal and postprandial serum gastrin concentrations in patients with duodenal ulcer. Clin Pharmacol Ther 1988;43:643–647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Table illustrating the mean esophageal and gastric pH, and the frequency, type, and height of the reflux events in 9 of 27 apparently healthy cats that had ≥ 1 reflux event during anesthesia for an elective dental procedure.


