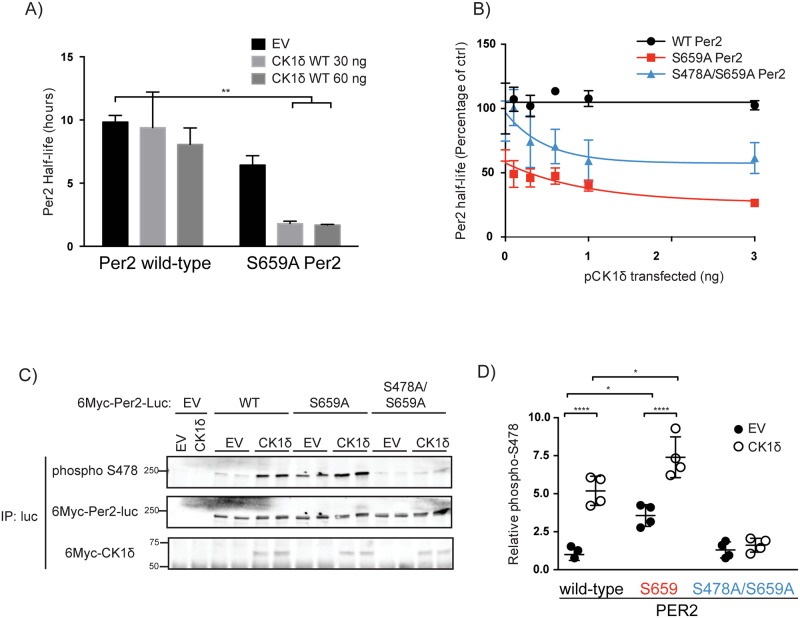
Fig 1. CK1δ mediate increased degradation of PER2(S659A) through phosphorylation at S478.
A) The stability of PER2(S659A) is sensitive to CK1δ activity. 50 ng of plasmid encoding wild-type or S659A PER2::LUC was co-transfected into HEK293 cells with indicated amount of empty vector (EV), or pCK1δ. Cycloheximide was added 20 hours post-transfection and the half-life of PER2::LUC was calculated using Graphpad Prism’s one-phase decay algorithm. B) S478A mutation partially rescues PER2(S659A) instability. 10 ng of wildtype, S659A or S478A/S659A double mutant were co-transfected into HEK293 cells with indicated amount of pCK1δ. Cycloheximide was added 20 hours post-transfection and the half-life of PER2::LUC was calculated as above. Half-life shown is plotted as a percentage, where the half-lives for samples with no pCK1δ co-expressed were set as 100%. C, D) Mutation of S659 increases phosphorylation of the β-TrCP site in a CK1δ sensitive manner. 6Myc-tagged PER2::LUC was co-expressed in HEK293 cells with empty vector (EV) or 6Myc-tagged pCK1δ in the presence of β-TrCP(ΔFbox) to prevent PER2 degradation. PER2::LUC was immunoprecipitated using an antibody targeting firefly luciferase, and immunoblotted using the indicated antibodies. Quantification of three independent experiments done in duplicates by densitometry is shown in D, where phospho-S478 is normalized to total PER2.