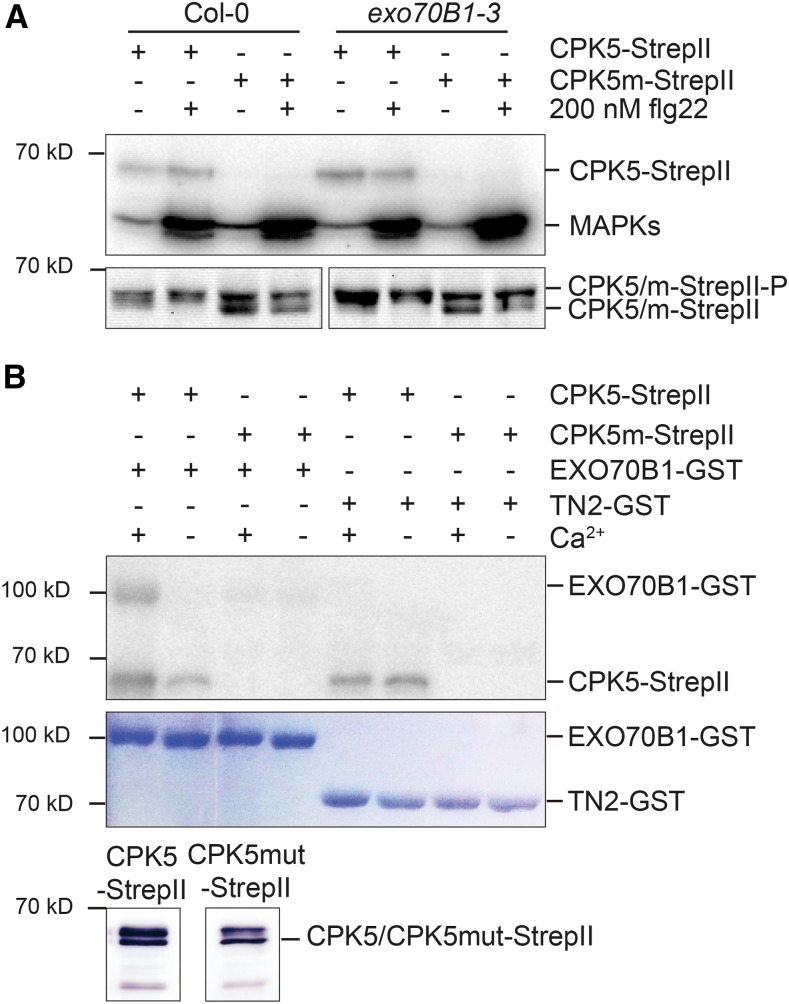
Figure 7.
CPK5 Shows Enhanced Biochemical Activity in exo70B1-3 and Can Phosphorylate EXO70B1 but Not TN2.
(A) In-gel kinase assay. CPK5 is active in exo70B1-3 in the absence of flg22 treatment. Protoplasts were isolated from 6-week-old Col-0 and exo70B1-3 plants and transfected with CPK5-StrepII and kinase-deficient CPK5m-StrepII before elicitation with either buffer (−) or 200 nM flg22 (+) for 15 min. Total protein was separated by SDS-PAGE and subjected to an in-gel kinase assay using myelin basic protein as substrate or to immunoblotting. The proteins were visualized by autoradiography (upper panel) or analyzed by immunostaining with Strep-Tactin HRP (lower panel). The experiment was performed three times with similar results.
(B) In vitro kinase assay. CPK5 phosphorylates EXO70B1 but not TN2 in vitro. CPK5-StrepII and CPK5m-StrepII were transiently expressed in N. benthamiana leaves, and affinity purification was controlled by immunoblotting (lower panel). The autophosphorylation activity of CPK5-StrepII and CPK5m-StrepII and activity toward recombinant substrate proteins EXO70B1-GST and TN2-GST were assessed in the presence of calcium or EGTA as indicated. Phosphorylation was visualized by autoradiography (upper panel) and the amount of substrate protein was visualized by Coomassie staining (lower panel). The experiment was performed four times with similar results.