Figure 1.
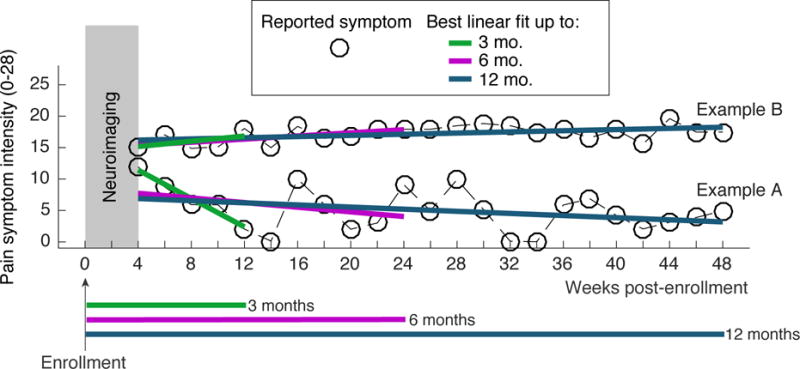
Data from two example UCPPS patients illustrating the design of the study and outcome measures. Within 4 weeks of enrollment, each UCPPS patient underwent neuroimaging procedures that captured the resting state fMRI (rs-fMRI) data. UCPPS symptoms, both pain symptom severity and urinary symptom severity, were assessed every 2 weeks for 48 weeks (12 4-week months). Symptoms during the first 4 weeks of the study were not analyzed to allow for a run-in period. Linear regression was used to quantify the trend in symptoms over a 3-month (12 week) period, a 6 month (24 week) period, and a 12 month (48 week) period. rs-fMRI measures were used to predict symptom change across the population of UCPPS patients studied. Example patient A exhibited a rapid pain symptom decrease in the first 3-months after neuroimaging and will be part of a group termed “improvers” for the 3-month time period. Example patient B does not exhibit 3-month symptom change and will be part of a group termed “non-improvers” for the 3-month time period.
