Figure 3. The LMPTP inhibitor series is selective and displays an uncompetitive mechanism of action.
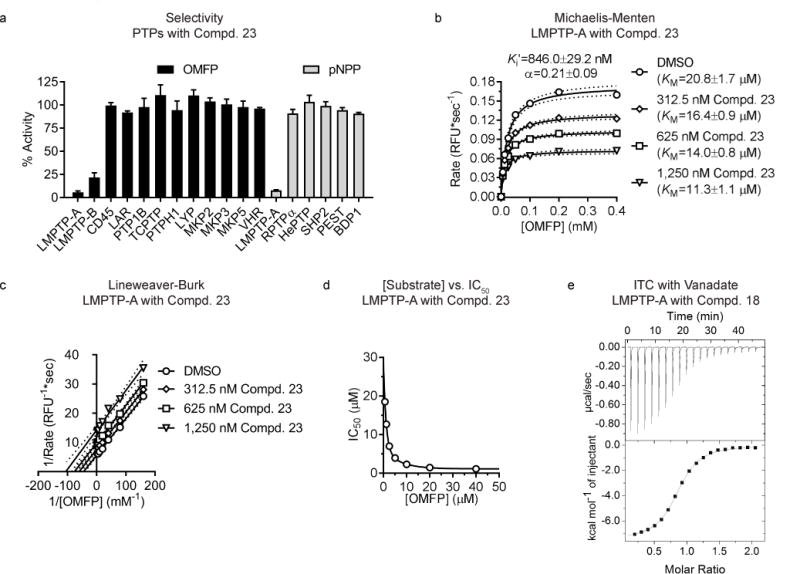
(a) Protein tyrosine phosphatases (PTPs) were incubated with 0.4 mM 3-O-methylfluorescein phosphate (OMFP) or 5 mM para-nitrophenylphosphate (pNPP) in the presence of dimethylsulfoxide (DMSO) or 40 μM Compd. 23. Mean±SEM % activity of PTPs incubated with Compd. 23 compared to DMSO from 3 independent experiments is shown. For each PTP, units of activity equivalent to 10 nM LMPTP-A were used. (b–c) Activity of 0.78 nM human LMPTP-A on increasing OMFP concentrations in the presence of increasing Compd. 23 concentrations. Data from 2 independent experiments performed in quadruplicate. (b) Mean reaction rate vs. OMFP concentration is shown. Lines show fitting to the Michaelis-Menten equation with 95% confidence intervals. (c) Lineweaver-Burk plot of data from (b). Lines show fitting to linear regression with 95% confidence intervals. (d) IC50 values were calculated for Compd. 23 on 0.78 nM human LMPTP-A-catalyzed hydrolysis of increasing concentrations of OMFP. Mean IC50 from 2 independent experiments performed in quadruplicate is shown. Lines show fitting to 2-phase decay. (e) Isothermal calorimetry (ITC) was performed on human LMPTP-A titrated with increasing Compd. 18 concentrations in the presence (3 replicates) or absence (4 replicates) of 200 μM sodium orthovanadate. Representative titration image in the presence of vanadate.
