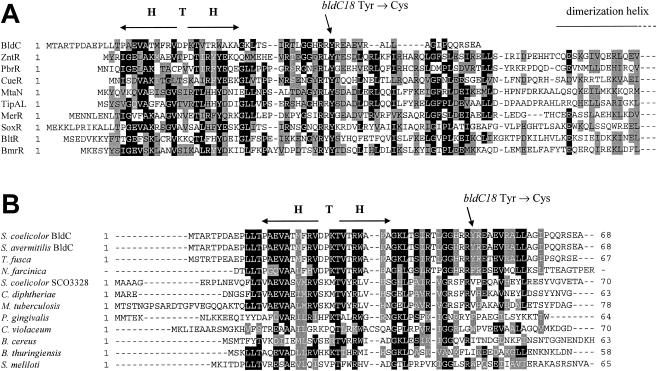
FIG. 3.
Amino acid sequence alignments. (A) Alignment of BldC with the N-terminal DNA-binding domains of MerR family proteins. Note that the complete BldC sequence is shown but that the MerR family proteins are arbitrarily truncated halfway through the dimerization helix. Proteins and their corresponding NCBI accession numbers are as follows: S. coelicolor BldC (CAB56371), E. coli ZntR (BAB37580), Ralstonia metallidurans PbrR (CAC28872), E. coli CueR (AAC73589), Bacillus subtilis MtaN (CAB15677), S. lividans TipAL (CAB42766), Pseudomonas aeruginosa MerR (CAA83897), E. coli SoxR (CAA42162), B. subtilis BltR (CAB14599), and B. subtilis BmrR (CAB14333). (B) Alignment of BldC with other members of the BldC family of small DNA-binding proteins. The complete sequence of each protein is shown. Proteins and their corresponding NCBI accession numbers are as follows: S. coelicolor BldC (CAB56371), S. avermitilis BldC (= SAV4130) (BAC71842), T. fusca (ZP_00293028), N. farcinica (partial sequence), S. coelicolor SCO3328 (CAB45362), C. diphtheriae (CAE48899), M. tuberculosis (CAE55290), P. gingivalis (AAQ66571), C. violaceum (AAQ61522), B. cereus (AAP12345), B. thuringiensis bacteriophage Bam35c (CAD59942), and S. meliloti (CAC41930). H T H, helix-turn-helix motif. The position of the BldC Y49C amino acid substitution caused by the bldC18 point mutation is shown.