Figure 1.
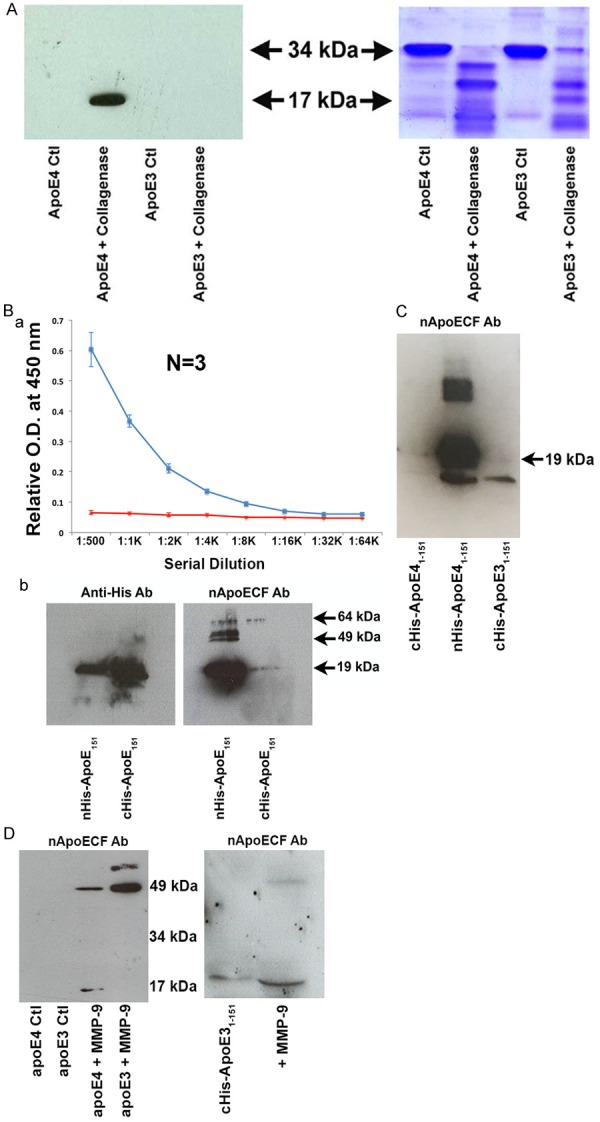
Characterization of a novel site-directed cleavage antibody to fragmented apoE4. (A) Preincubation of human recombinant apoE4 but not apoE3 with Type I collagenase for 1.5 hours at 37°C results in the generation of a 17 kDa fragment recognized by affinity-purified nApoECFp17 antibody (lane 2) following Western blot analysis. The right panel depicts the transferred gel slab that was stained with Coomassie blue revealing full-length apoE4 and E3 (34 kDa) as well as the associated fragmentation pattern following incubation with Type I collagenase. (B) Specificity of nApoECFp17 to the C-terminal end of apoE41-151. Top panel: ELISA assays in which 96-well plates were coated overnight at 4°C with either apoE41-151 with the His-Tag attached to the C-terminal end (red line), or with the His-Tag attached to the amino-terminal end (blue line). The results indicated that the nApoECFp17 antibody only recognized apoE41-151 in which the His-tag was attached to the amino-terminal end. Similar experiments as shown in the top panel for ELISA results were performed using Western blot analysis. In this case, the nApoECFp17 antibody (1:500 dilution) preferentially immunolabeled the apoE4 fragment in which the His-tag was localized to the amino-terminal end (ride side of bottom panel). The left panel shows the identical experiment in utilizing an anti-His antibody that easily labeled both protein fragments regardless of the location of the His-tag. (C) Specificity of nApoECFp17 antibody to the C-terminal end of apoE41-151. Identical Western blot experiments as presented in Panel Bb with the exception that an amino-terminal fragment of apoE31-151 with the His-tag located on the C-terminal end was included as a comparison. The results show that the nApoECFp17 antibody preferentially immunolabeled only the apoE fragment in which the His-tag was localized to the amino-terminal end (middle panel). For both (B and C) the following amounts of protein were loaded on SDS gels: cHis-ApoE41-151, 72 µg, cHis-ApoE31-151, 72 µg, and nHis-ApoE41-151, 29 µg. (D) Left Panel, Full-length human recombinant apoE3 or apoE4 was incubated with activated MMP-9 for 24 hours at 37°C and reactions were terminated by the addition of 5X sample buffer. Proteins were separated on 15% SDS-PAGE gels, transferred to nitrocellulose, and then probed with the nApoECFp17 overnight at 1:500. The “Ctl” lanes represent full-length apoE4 or E3 proteins incubated in the absence of MMP-9. Generation of the p17 band and a high-molecular weight band at ~49 kDa was observed following incubation of full-length apoE4 with MMP-9, while only a high molecular weight band ~49 kDa was observed following cleavage of full-length apoE3. The right panel depicts a similar experiment using the cHis-ApoE31-151 fragment in which the 6× His tag was coupled to the C-terminal end (lane 1). Incubation of cHis-ApoE31-151 with active MMP-9 (lane 2) resulted in a stronger and slightly smaller p17 band. These results suggest that cleavage of the 6× His tag by MMP-9 produced a slightly smaller fragment that now lacking the 6×-His tag allowed for better access of the epitope for the nApoECF antibody and stronger immunolabeling (see right panel D lane marked “+MMP-9”).
