Figure 2.
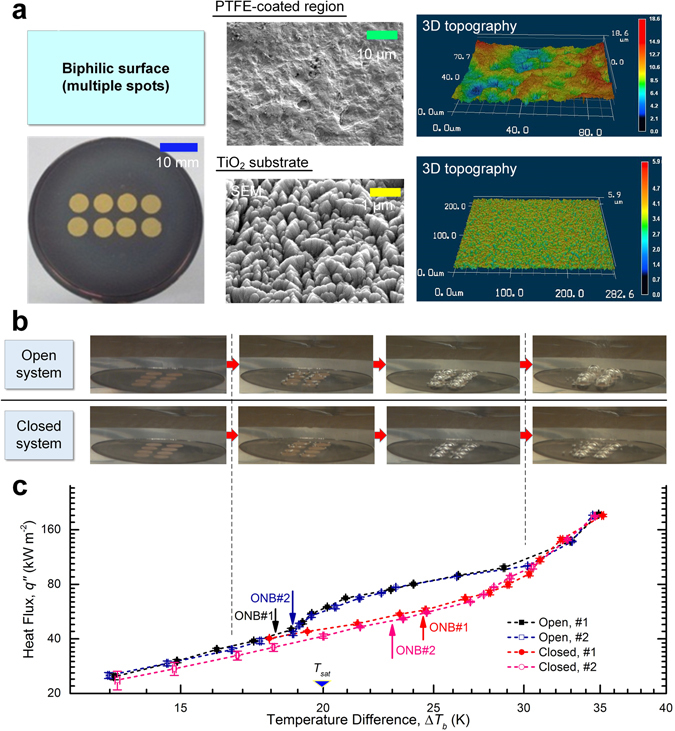
Effect of dissolved gas on boiling heat transfer on a biphilic surface. (a) A heterogeneous surface with mixed wettability. The superhydrophilic TiO2 substrate (after 12 hours of UV irradiation, θ e ≈ 0°) was coated with an array of hydrophobic (θ e > 120°) PTFE circular spots of 6 mm in diameter and 7 mm in pitch. The SEM and 3D laser profilometer images reveal contrasting surface topographies between the TiO2 and PTFE subregions, which had an average roughness of 0.3 µm and 3 µm, respectively. (b) Evolution of the boiling characteristics. Following the initial quiescent stage of natural convection, small bubbles first appeared around the interface between the hydrophilic and hydrophobic subregions, which then coalesced and formed a single bubble completely encompassing each hydrophobic island. Although the expansion of the bubble base was limited to the edge of the hydrophobic coatings, the mostly stationary bubbles grew notably larger in the open system due to the presence of dissolved gas. At high superheats, more bubbles started to nucleate on the TiO2 surface as well, which quickly departed from the surface. (c) Plot of the boiling curves. The open and closed cases differed in the incipience of boiling: ΔT sat = 4.6 K (run #1) and 2.9 K (run #2) in the closed system, compared with ΔT sat = −1.6 K (run #1) and −1.4 K (run #2) in the open system. Error bars represent standard deviations of the least-square fittings. An increasing divide seems to emerge between the boiling curves before re-converging in the regime of fully-developed nucleate boiling, which is in large part attributable to particularly strong dissolved gas-induced thermocapillary convection under the open condition.
