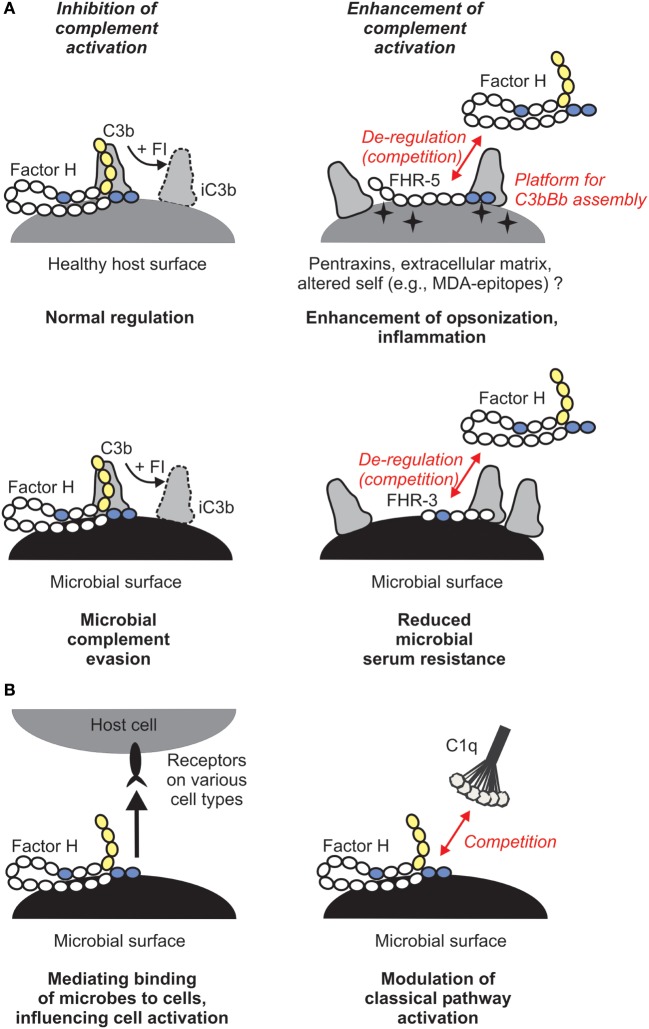
Figure 2.
Role of the human factor H (FH) protein family in microbial immune evasion. (A) In addition to its role as plasma complement inhibitor, FH recognizes and binds to host surfaces and protects them from complement attack. Several microbes exploit this mechanism and recruit FH to their surface in order to escape from the complement system. FH-related (FHR) proteins may bind to certain host ligands or altered host surfaces that are exposed during inflammation or tissue damage (such as pentraxins, extracellular matrix proteins, or oxidative modifications of lipids) and displace FH, resulting in increased opsonization. FHRs may act as decoys and compete with FH for binding to microbial proteins. For example, FHR-3 was described to inhibit binding of FH to fHbp of Neisseria meningitidis. (B) FH was described to have additional functions. By simultaneously binding to certain microbes and receptors (such as CR3) on host cells, it may facilitate uptake of the microbe by immune cells and modulate cell activation, or facilitate entry of microbes into epithelial cells (left panel). FH was also shown to inhibit binding of C1q to apoptotic cells and E. coli and thus may modulate classical pathway activation and opsonization (right panel).