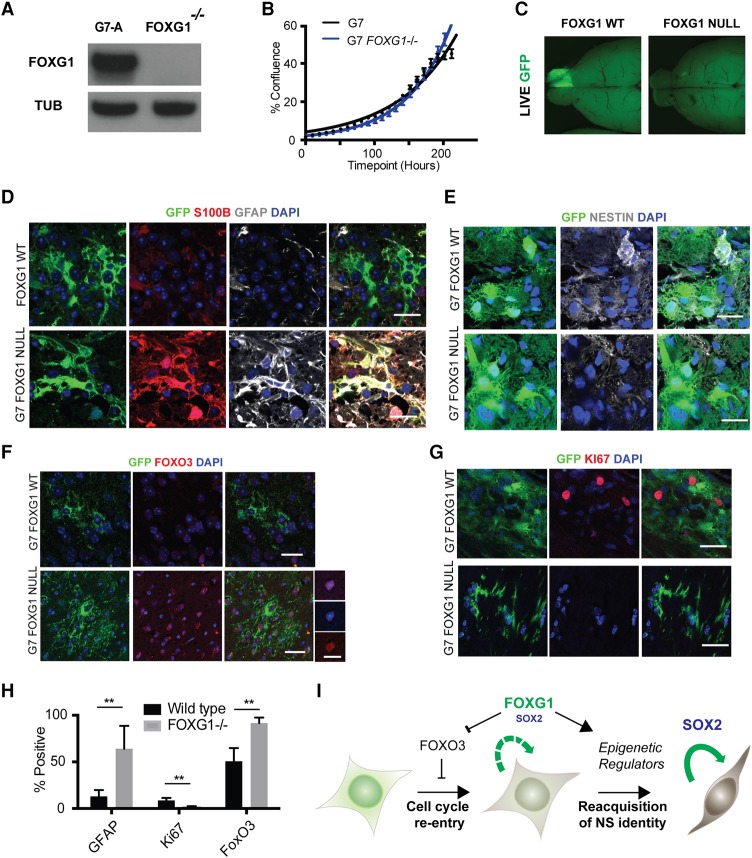
Figure 7.
Genetic ablation of FOXG1 in human GBM stem cells using Cas9-assisted gene targeting. (A) CRISPR/Cas-based gene targeting was used to knock out FOXG1 in G7 cells, and no protein was detectable by Western blot, with a frameshift mutation demonstrated on the second FOXG1 allele in this clone (see Supplemental Fig. S7). (B) Growth curve displaying percentage confluence over time for G7 and G7 FOXG1−/− cell lines, indicating that the FOXG1−/− clone proliferates at a rate similar to that of parental controls in vitro. (C) Upon xenotransplantation, wild-type G7 cells expressing a GFP reporter form invasive tumors, but FOXG1−/− derivatives fail to do so. n = 4 for each cell line. (D) Immunohistochemistry (IHC) analysis of xenografts reveals that the G7 FOXG1 mutant cells display increased expression of astrocyte markers S100β (red) and GFAP (gray), reduced expression of NESTIN (gray) (E), increased expression of FOXO3 (F), and decreased expression of Ki67 (red) (G). (H) Quantitation of the percentage of cells positive for GFAP, Ki67, and FOXO3 from IHC. (I) Working model of FOXG1 and SOX2 function in GBM based on this study. (Green cell) Post-mitotic or quiescent astrocytes; (brown/gray cell) radial glia-like proliferative NS cell. Bar, 10 µm; bar for higher-magnification images in F, 20 µm. Students t-test, n = 4; P < 0.005.