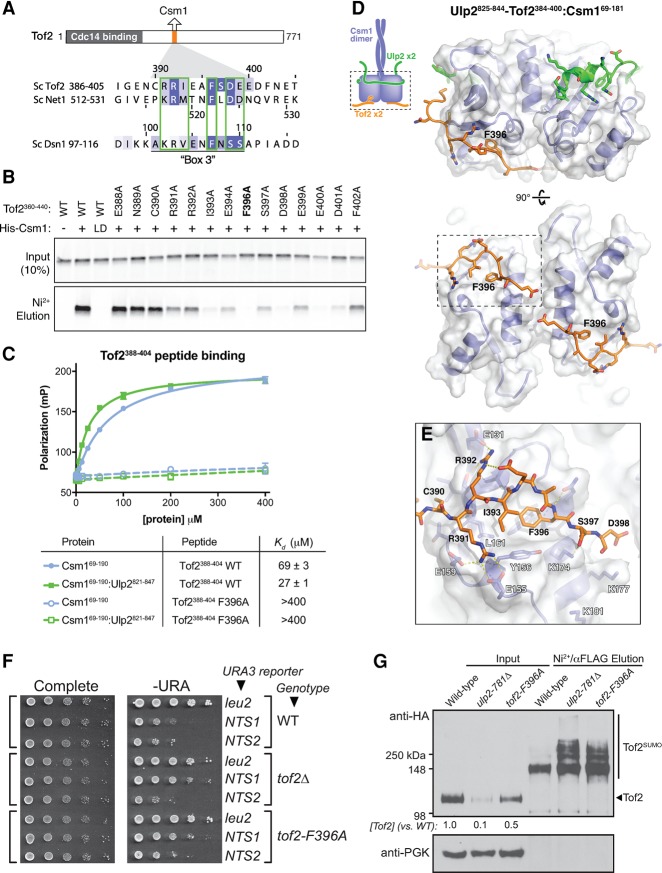
Figure 6.
A conserved motif in Tof2 and Net1 binds the conserved hydrophobic surface of Csm1. (A) Schematic of the Cdc14-binding (residues 1–270) (Waples et al. 2009) and Csm1-binding (residues 390–400) (see Supplemental Fig. S5) regions of Tof2 and the sequence of the Csm1-binding region from Tof2 aligned with equivalent regions in Net1 and Dsn1. (B) Pull-down assay showing the effects of Tof2 point mutations on Tof2–Csm1 binding. Binding is disrupted by the Csm1 L161D mutation (LD), implicating the Csm1 conserved hydrophobic surface in Tof2 interaction. We observed similar results with the equivalent region of Net1, including the disruption of binding by the Csm1 L161D mutation (Supplemental Fig. 5A). (C) Fluorescence polarization assay measuring binding of Csm169–190 (blue) or Ulp2821–847:Csm169–190 (green) to a fluorescently labeled Tof2388–404 peptide (Tof2 wild type; solid lines) or the same peptide with an F396A mutation (Tof2 F396A; dashed lines). See Supplemental Figure 5B for binding of Ulp2825–844–Tof2384–400:Csm169–190 to these peptides. (D) Side and bottom views of the Csm169–181:Ulp2825–844–Tof2384–400 structure, with Csm1 in blue, Ulp2 in green, and Tof2 in orange. (E) Close-up view of Tof2's interaction with the Csm1 conserved hydrophobic surface. (F) The effect of Csm1 and Tof2 point mutations on rDNA silencing. (G) The effect of Csm1 and Tof2 point mutations on Tof2 sumoylation and abundance following purification of total sumoylated proteins for Western blot analysis as indicated.