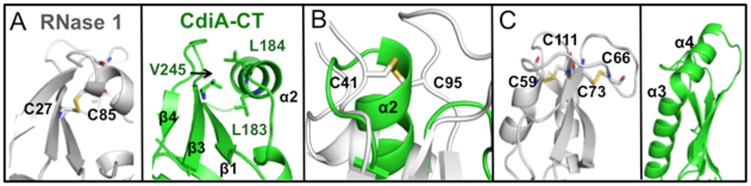
Figure 7.
Structural comparison of mouse RNase 1 and CdiA-CTYkris. For the three panels, Cartoon representation depicts CdiA-CTYkris (green) and mouse RNase 1 (white, PDB code: 3TSR) with disulfide bonds represented in sticks with carbon and sulfur atoms colored white and yellow, respectively. (A) The Cys27–Cys85 bond in RNase 1 (left) is substituted by hydrophobic interactions in CdiA-CTYkris (right). (B) Superimposition of RNase 1 and CdiA-CTYkris. The disulfide bond between Cys41 and Cys96 in RNase 1 is replaced by an α-helix in CdiA-CTYkris. (C) RNase 1 has an extended loop that is stabilized by Cys58–Cys111 and Cys66–Cys73 linkages (left). This loop region is substituted with a fourth α-helix in the CdiA-CTYkris structure (right).