Figure 1.
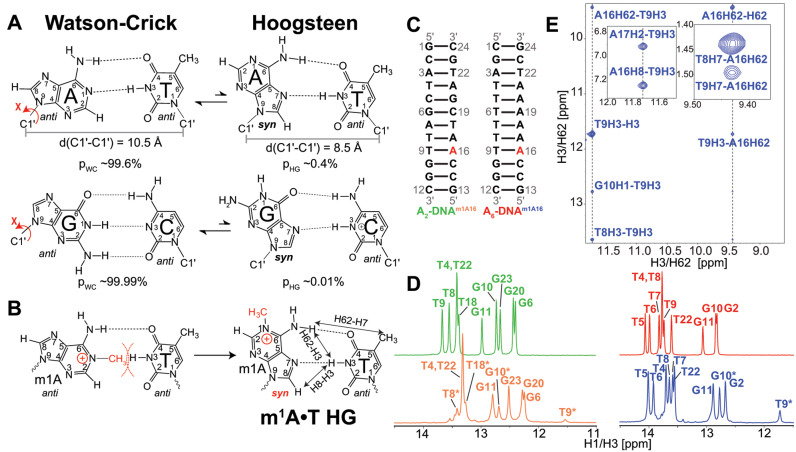
(A) Watson–Crick (WC) base pairs (bps) exists in dynamic equilibrium with transient low-abundance Hoogsteen (HG) bps through flipping of the purine base 180° about the glycosidic bond from anti to syn conformation. This is also accompanied by a net reduction of C1΄–C1΄ distance by ∼2.0–2.5 Å. Shown are the average populations (pWC/pHG) for WC and HG bps as measured by nuclear magnetic resonance (NMR) relaxation dispersion (RD) methods (33,35). (B) m1A stabilizes HG bp due to steric clash between the methyl group at m1A-N1 position and T-H3. Nuclear Overhauser effect (NOE) distance connectivity between complementary nucleotides that help in distinguishing HG, WC and reverse-HG pairing modes are indicated using arrows. (C) A2- and A6-DNA duplexes used in this study. (D) 1D imino 1H spectra of A2-DNA (green), A2-DNAm1A16 (orange), A6-DNA (red) and A6-DNAm1A16 (blue). Spectra for for A2-DNA/A6-DNA and A2-DNAm1A16/A6-DNAm1A16 were recorded at 25 and 9°C, respectively. Sites experiencing exchange broadening are denoted with an asterisk (*). (E) T9-H3–A16-H8, T9-H3–A-H62 and T9-H7–A-H62 NOE connectivity distinguishes the formation of HG bp from other pairing modes in A6-DNAm1A16 (19) (see Supplementary Figure S3 for A2-DNAm1A16). Sequential imino NOEs between T9-H3 with T8-H3 and G10-H1 indicate the formation of a stable duplex.