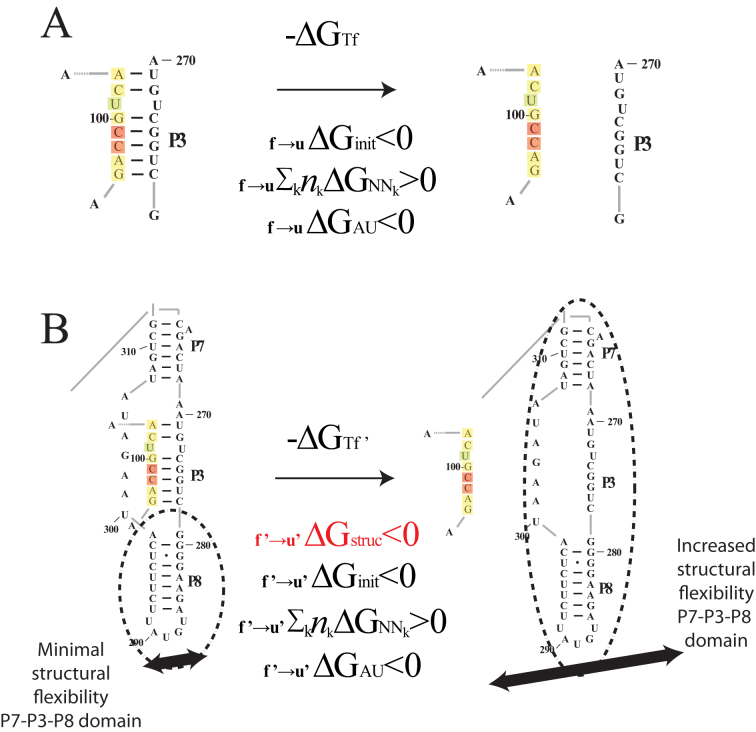
Figure 2.
Proposed regional availability factor in novel biophysical model. The influence of structural availability (as captured by the ensemble of suboptimal structures) on intermolecular interactions can be attributed to variable entropic contributions to asRNA-target binding free energies that depend on structural context of targeted regions. The f →u subscript denotes the change from folded to unfolded states and the apostrophe represents the adjusted free energy accounting for regional availability. The init, AU and NNk subscripts indicate the standard free energy terms associated with initiation, terminal AU pairs and stacking free energies (respectively) in the standard nearest neighbor model. (A) The thermodynamic model from Figure 1 captures the nearest neighbor free energy of unfolding the target region based on the minimum free energy (MFE) structure. (B) The proposed regional availability factor (estimated as the sum of one minus the base pairing probabilities in the target region calculated from the ensemble of suboptimal structures in Nupack (64)) captures entropic effects of target RNA distal structural variations that occur upon target unfolding. As an example, there is a potential for a significant portion of the gI intron (regions P7, P3, P8) to have increased vibrational and translational degrees of freedom upon breaking of the long-range tertiary base pairs in the highlighted target region of the P3 helix, an effect which is captured by this example suboptimal structure. Furthermore, with the use of in vivo optimization, the effects of entropy contributions from interactions with unknown cellular factors in these dynamic regions may also be captured by the ensemble of suboptimal structures from Nupack.