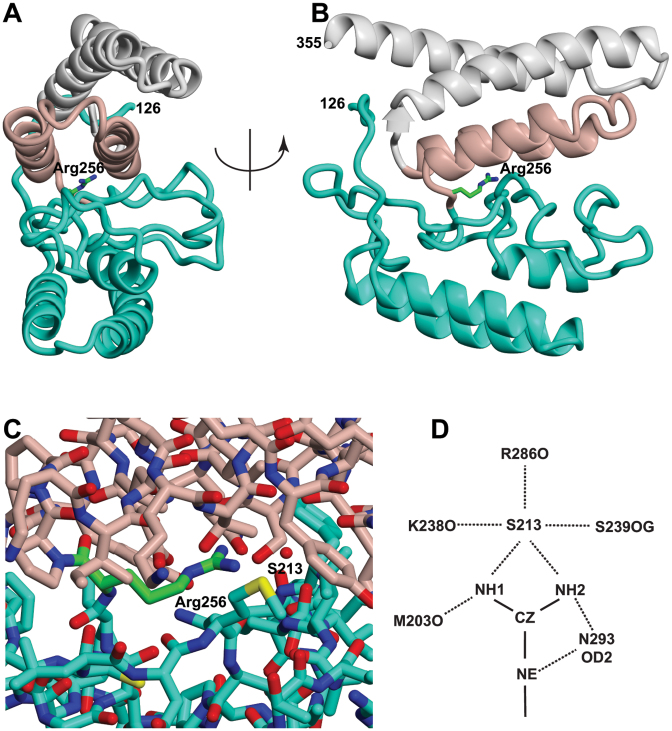
Figure 3.
Arg256 is buried in the interface between the Sac3 TPR helices and the three additional helices. (A and B) Illustration of how Sac3 Arg256 is sandwiched between the two helices of the TPR repeat that contains residues 255–269 and 280–296 (pink) and the additional module (residues 126–250, green). Arg256 and the Sac3 255–269 and 280–296 helices formed lattice contacts in previous crystal structures and were proposed to form an interaction interface (15), but in the more complete structure of the Sac360-556:Thp1:Sem1 complex they are buried and inaccessible. (C) Detail of the residues surrounding Arg256 and water S213 that are buried in the interface between the 255–296 TPR-like repeat and residues 125–250. (D) Hydrogen bond network linking Arg256 to Met203, Lys238, Arg286, Ser289, Asn293 and water S213. See also Supplementary Movies 2 and 3.