Figure 7.
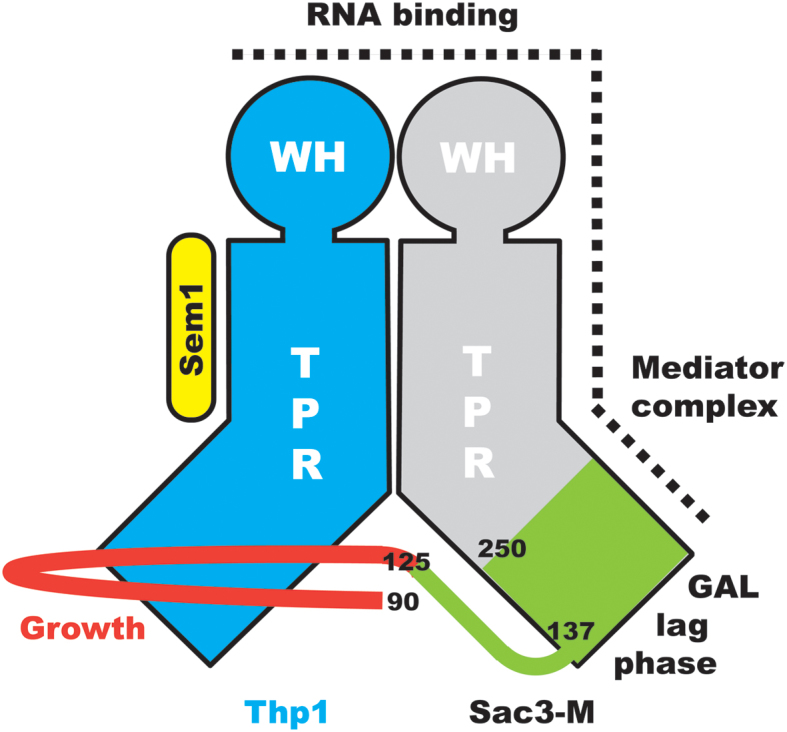
Schematic illustration of the structure of the TREX-2 M-region. Both Sac3 and Thp1 have PCI folds based on a series of α-helical TPR repeats together with a winged helix (WH) domain, whereas Sem1 lies along one side of Thp1. The structure of the Sac360-556:Thp1:Sem1 complex has identified two new structural features in Sac3: an extension of the TPR region from residue 250 to residue 127 (green) and an extended loop comprising residues 90–125 that links the distal portion of the Sac3 TPR region with Thp1. A cluster of positively-charged residues that extends from the Thp1 winged helix domain and down one side of Sac3 appears to be important for binding RNA. The extended loop (residues 90–125) is important for growth, whereas residues 126–250 are important for the induction of GAL genes when cells are moved to galactose as a carbon source. Other studies (15) have indicated that positive residues in the Sac3 TPR region also function in conjunction with the mediator complex.
