Abstract
Background
Hemodialysis (HD) patients frequently suffer from severe anemia caused by various hemorrhagic disorders in addition to renal anemia. Intradialytic blood transfusion is sometimes performed; however, the cerebral oxygenation changes associated with this procedure remain unclear.
Methods
Sixteen HD patients with severe anemia who required intradialytic blood transfusion were included (12 men and 4 women; mean age, 64.8 ± 9.8 years). Cerebral regional oxygen saturation (rSO2) was monitored using near-infrared spectroscopy, and cerebral fractional oxygen extraction (FOE) was calculated before and after HD. Twenty-five HD patients with well-maintained hemoglobin (Hb) levels were included as a control group.
Results
Cerebral rSO2 values were significantly lower in HD patients with severe anemia than in the control group (42.4 ± 9.9 vs. 52.5 ± 8.5%, p = 0.001). Following intradialytic blood transfusion (385 ± 140 mL of concentrated red blood cells), Hb levels significantly increased (from 7.2 ± 0.9 to 9.1 ± 1.1 g/dL, p < 0.001), and cerebral rSO2 values significantly improved after HD (from 42.4 ± 9.9 to 46.3 ± 9.0%, p < 0.001). Cerebral FOE values before HD in patients with severe anemia were significantly higher than those in the control group (severe anemia, 0.56 ± 0.10; controls, 0.45 ± 0.08; p < 0.001). After HD with intradialytic blood transfusion, these values significantly decreased (0.52 ± 0.09 after HD versus 0.56 ± 0.10 before HD, p = 0.002).
Conclusion
HD patients with severe anemia represented cerebral oxygen metabolism deterioration, which could be significantly improved by intradialytic blood transfusion.
Keywords: Anemia, Hemodialysis, Red blood cells, Transfusion, Hemoglobin, Cerebral oxygenation, Near-infrared spectroscopy
Introduction
Renal anemia is one of the usual complications in patients with advanced chronic kidney disease, including those on hemodialysis (HD). In the United States in 1989 and in Japan in 1990, the use of erythropoiesis-stimulating agents in clinical settings was approved, which dramatically improved hemoglobin (Hb) levels in patients with advanced chronic kidney disease and on HD. With erythropoiesis-stimulating agent administration inducing an Hb increase, the necessity for blood transfusion has been reduced [1]. Despite this, HD patients frequently suffer from severe anemia caused by various hemorrhagic disorders [2]; therefore, intradialytic blood transfusion is sometimes performed to improve Hb levels in HD patients with severe anemia. Recently, near-infrared spectroscopy (NIRS) has been used to measure regional oxygen saturation (rSO2), which is a real-time tissue oxygenation marker [3, 4, 5]. In particular, cerebral rSO2 values were significantly lower in HD patients than in healthy controls [6, 7], and the Hb increase induced by ultrafiltration during HD did not lead to improvement of cerebral rSO2 values [7, 8]. However, to date, few reports have examined the association between intradialytic blood transfusion and cerebral oxygenation; therefore, changes in cerebral oxygenation associated with intradialytic blood transfusion in HD patients with severe anemia remain unclear. In this study, we focused on the cerebral oxygenation of HD patients with severe anemia, and to confirm the effect of intradialytic blood transfusion on cerebral oxygenation, we compared the changes in cerebral rSO2 and fractional oxygen extraction (FOE) values [9, 10] before and after HD between severe anemic HD patients with intradialytic blood transfusion and ultrafiltration, and not just anemic HD patients with only ultrafiltration as a control group.
Subjects and Methods
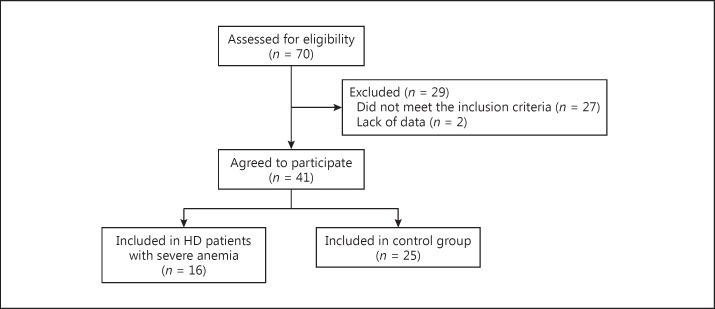
In this study, we included patients with severe anemia undergoing HD who met the following criteria: (1) patients with end-stage renal disease receiving intermittent HD, (2) severe anemic patients requiring blood transfusion, which was caused by hemorrhagic disorders, including gastrointestinal bleeding or operation-related anemia, in addition to renal anemia, and (3) patients who received intradialytic blood transfusion to improve severe anemia out of necessity as determined by a medical practitioner. Furthermore, HD patients with well-maintained Hb levels who met the following criteria were included and assigned to the control group: (1) patients who had started HD at least 3 months prior, (2) patients with end-stage renal disease receiving intermittent HD, and (3) patients with Hb levels >10 g/dL before HD. The exclusion criteria included severe cardiovascular disease, including congestive heart failure with massive pleural effusion or any uncontrolled chronic condition, severe cerebrovascular disease, cognitive impairment, and lung disease with shortness of breath at rest. Figure 1 shows a flowchart of patient enrollment and analysis.
Fig. 1.
Flowchart of patient enrollment and analysis. HD, hemodialysis.
The HD patients with severe anemia who underwent intradialytic blood transfusion consisted of 16 subjects (12 men and 4 women; mean age, 64.8 ± 9.8 years; mean HD duration, 4.3 ± 6.9 years). Their causes of chronic renal failure were type 2 diabetes mellitus (7 patients), nephrosclerosis (4 patients), chronic glomerulonephritis (1 patient), and others (4 patients). In addition, their causes of severe anemia were gastrointestinal bleeding (5 patients), renal anemia (4 patients), operation-related anemia (2 patients), inflammation-related anemia (2 patients), and other hemorrhagic disorders (3 patients). The control group consisted of 25 HD patients who did not undergo intradialytic blood transfusion (18 men and 7 women; mean age, 67.2 ± 9.5 years; mean HD duration, 4.6 ± 4.4 years). Their causes of chronic renal failure were type 2 diabetes mellitus (16 patients), nephrosclerosis (2 patients), chronic glomerulonephritis (5 patients), and others (2 patients). Each patient received maintenance HD 2 or 3 times per week, with each HD session lasting >3 h. The HD dialysate was composed of 140 mEq/L Na+, 2.0 mEq/L K+, 110.0 mEq/L Cl–, 3.0 mEq/L Ca2+, 1.0 mEq/L Mg2+, 30 mEq/L HCO3–, and 100 mg/dL glucose. Dialysate purification was evaluated as recommended in the Japanese Society for Dialysis Therapy guidelines [11]. The bacteria count and endotoxin concentrations in the dialysate in this study were <0.1 CFU/mL and <0.001 EU/mL, respectively. The patients’ general baseline characteristics are summarized in Table 1. There were no significant differences in comorbidities, including cardiovascular and cerebrovascular diseases, between the control group and HD patients with severe anemia. Furthermore, there were no significant differences in dry weight, ultrafiltration rate, and HD time between the 2 groups. The dose of erythropoiesis-stimulating agent was significantly higher in the HD patients with severe anemia than in the control group, whereas there was no difference in number of patients receiving iron administration between the 2 groups. All patients gave informed consent to participate in this study, which was approved by the Institutional Review Board of Saitama Medical Center, Jichi Medical University, Japan (RIN 14–114). The study also conformed to the provisions of the Declaration of Helsinki (as revised in Tokyo in 2004).
Table 1.
Comparison of the baseline characteristics between the control group and HD patients with severe anemia
| Controls | HD patients with severe anemia | p value | |
|---|---|---|---|
| Patients | 25 | 16 | |
| Sex (male/female) | 18/7 | 12/4 | 0.881 |
| Age, years | 67.2±9.5 | 64.8±9.8 | 0.435 |
| HD duration, years | 4.6±4.4 | 4.3±6.9 | 0.868 |
| Primary disease | |||
| Diabetic nephropathy | 16 | 7 | 0.120 |
| Nephrosclerosis | 2 | 4 | |
| Chronic glomerulonephritis | 5 | 1 | |
| Others | 2 | 4 | |
| Comorbidities | |||
| Cardiovascular disease | 8 (32.0%) | 8 (50.0%) | 0.410 |
| Cerebrovascular disease | 10 (40.0%) | 3 (18.8%) | 0.279 |
| HD | |||
| Dry weight, kg | 56.8±12.4 | 58.3±8.6 | 0.686 |
| Ultrafiltration, mL/h | 550±201 | 502±237 | 0.484 |
| HD time, h | 3.8±0.4 | 3.7±0.4 | 0.410 |
| Causes of anemia | |||
| Gastrointestinal bleeding | 5 | ||
| Renal anemia | 4 | ||
| Operation-related anemia | 2 | ||
| Inflammation-related anemia | 2 | ||
| Other hemorrhagic disorders | 3 | ||
| Erythropoiesis-stimulating agent, IU/week | 4,560±3,343 | 10,188±6,537 | <0.001 |
| Iron administration (yes/no) | 3/22 | 2/14 | 0.962 |
| Blood transfusion volume, mL | 385±140 |
Values are presented as n, n (%), or mean ± standard deviation. HD, hemodialysis.
Patients’ Baseline Characteristics and Clinical Laboratory Measurements
We collected the patients’ baseline characteristics and other relevant data from their medical charts. The primary disease underlying the dialysis requirement, cardiovascular disease, and cerebrovascular disease were included from the medical records in our hospital. The causes of anemia were confirmed from the medical records and attending doctors.
Blood pressure and heart rate were measured with patients in the supine position before and after the HD sessions. In addition to ultrafiltration rate and HD time confirmation in all HD patients, intradialytic blood transfusion volume was confirmed in all HD patients with severe anemia. Blood samples were obtained at ambient temperature from the arteriovenous fistula of each patient before and after HD. Blood gas analysis, including arterial oxygen saturation (SaO2), was performed using the RAPIDLab 1265 blood gas analyzer (Siemens Healthineers, Tokyo, Japan).
Monitoring of Cerebral Oxygenation
Cerebral rSO2 was monitored at the forehead using the INVOS 5100C monitor (Covidien, Tokyo, Japan), which is based on the NIRS technology. This instrument uses a light-emitting diode which transmits near-infrared light at 2 wavelengths (735 and 810 nm), and 2 silicon photodiodes which act as light detectors. Results are read as a single numerical value that represents rSO2 [12, 13]. All data obtained by this instrument were immediately and automatically stored in sequence. Interobserver variance for this instrument, i.e., the reproducibility of the rSO2 measurements, is acceptable, as reported previously [14]. Therefore, rSO2 is considered reliable in estimating actual cerebral oxygenation levels.
Before HD, each patient rested in the supine position for >10 min. Next, measurement sensors were attached to the patient's forehead in the resting state. Thereafter, rSO2 was measured for 5 min before HD, and we evaluated the mean rSO2 value for 5 min as a cerebral oxygenation marker. In addition, we measured rSO2 values for 5 min after HD to confirm the change in cerebral rSO2 values after blood transfusion during HD. Furthermore, based on each cerebral rSO2 measurement, we calculated the cerebral FOE in each group before and after HD by using the following equation [9, 10]:
This variable reflects the balance between oxygen delivery and consumption; therefore, cerebral FOE changes before and after HD would reflect the changes in cerebral oxygen metabolism, which might be affected by the HD therapy itself or other factors, such as intradialytic blood transfusion.
Statistical Analysis
Data were expressed as mean ± standard deviation. The χ2 test was used to assess the associations among the patients’ baseline variables. The Student t test for paired or unpaired values was used in comparing the 2 groups. The comparison of clinical parameters, including cerebral rSO2, before and after HD was performed as matched pairs, resulting in a sufficient statistical power − 0.85 in HD patients with severe anemia and 0.97 in the control group –, although the statistical power in the comparison between the 2 groups as unpaired was 0.68, which was relatively low. All analyses were performed using IBM SPSS Statistics for Windows, version 19.0. A difference of p < 0.05 was considered significant.
Results
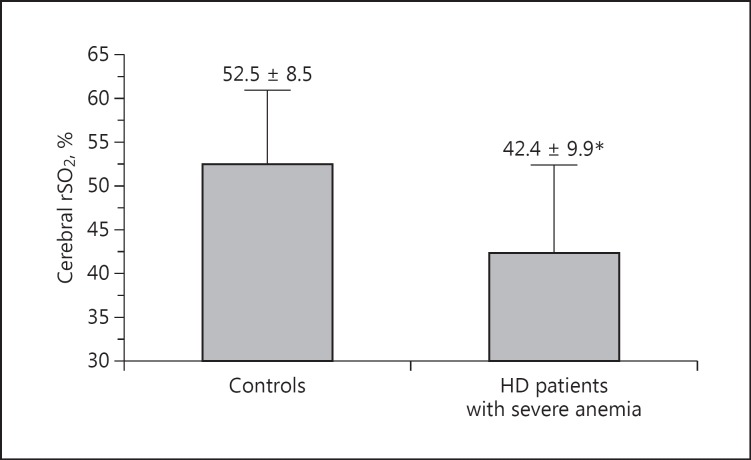
There was a significant difference in Hb levels before HD between the control group and HD patients with severe anemia (10.9 ± 0.7 and 7.2 ± 0.9 g/dL, p < 0.001; Table 2). In addition, there were significant differences in other clinical parameters (heart rate, serum creatinine, serum total protein concentration, serum albumin concentration, and C-reactive protein) between the 2 groups. Furthermore, cerebral rSO2 values before HD were significantly lower in HD patients with severe anemia than in the control group (controls, 52.5 ± 8.5%; HD patients with severe anemia, 42.4 ± 9.9%; p = 0.001 vs. controls; Fig. 2).
Table 2.
Differences in the clinical parameters before HD between the control group (n = 25) and HD patients with severe anemia (n = 16)
| Clinical parameters | Controls | HD patients with severe anemia | p value |
|---|---|---|---|
| Systolic BP, mm Hg | 143±19 | 133±22 | 0.125 |
| Diastolic BP, mm Hg | 73±14 | 71±14 | 0.621 |
| Heart rate, bpm | 70±11 | 81±14 | <0.001 |
| Hb, g/dL | 10.9±0.7 | 7.2±0.9 | <0.001 |
| pH | 7.36±0.05 | 7.39±0.06 | 0.159 |
| pO2, mm Hg | 82±14 | 91±17 | 0.112 |
| Oxygen saturation, % | 95.6±2.5 | 95.9±2.0 | 0.702 |
| Sodium, mEq/L | 137±3 | 136±3 | 0.112 |
| Potassium, mEq/L | 4.6±0.8 | 4.3±1.0 | 0.393 |
| BUN, mg/dL | 56±16 | 62±33 | 0.446 |
| Cr, mg/dL | 8.8±2.2 | 7.1±2.2 | 0.019 |
| Plasma glucose, mg/dL | 162±55 | 148±43 | 0.387 |
| Serum osmolality, mOsm/kg×H2O | 304±10 | 301±12 | 0.541 |
| Total protein, g/dL | 6.3±0.4 | 5.7±0.7 | <0.001 |
| Albumin, g/dL | 3.6±0.4 | 2.7±0.5 | <0.001 |
| C-reactive protein, mg/dL | 0.8±2.0 | 6.5±11.2 | 0.019 |
| Transferrin saturation, % | 27±20 | 29±31 | 0.784 |
| Serum ferritin, ng/mL | 85±55 | 323±510 | 0.065 |
Values are presented as mean ± standard deviation. BP, blood pressure; BUN, blood urea nitrogen; Hb, hemoglobin; HD, hemodialysis; pO2, partial pressure of oxygen.
Fig. 2.
Comparison of cerebral regional oxygen saturation (rSO2) values between the control group and hemodialysis (HD) patients with severe anemia. * p = 0.001 versus controls.
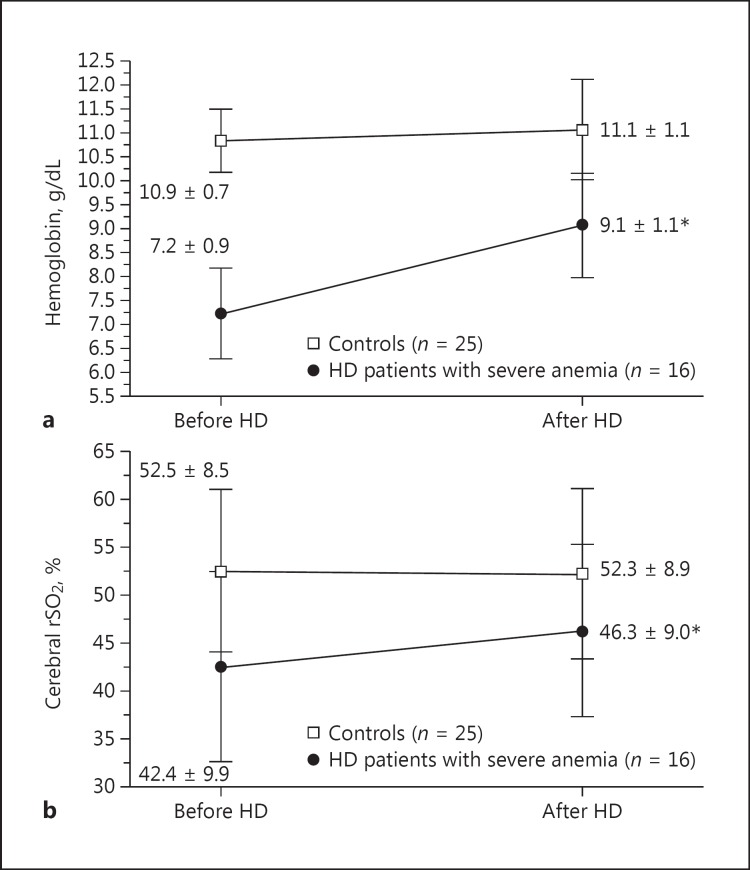
Intradialytic blood transfusion (385 ± 140 mL of concentrated red blood cells) was performed in patients undergoing HD with severe anemia in this study. Hb levels in HD patients with severe anemia significantly increased from 7.2 ± 0.9 to 9.1 ± 1.1 g/dL after HD (p < 0.001; Fig. 3a). In addition, cerebral rSO2 values significantly improved after HD compared to those before HD (from 42.4 ± 9.9 to 46.3 ± 9.0%, p < 0.001; Fig. 3b), whereas in the control group, cerebral rSO2 values did not change (from 52.5 ± 8.5 to 52.3 ± 8.9%, p = 0.436), with no significant Hb level changes before and after HD (from 10.9 ± 0.7 to 11.1 ± 1.1 g/dL, p = 0.296). Next, cerebral FOE values, which were calculated based on SaO2 and cerebral rSO2 results, were compared before and after HD between the control group and HD patients with severe anemia (Table 3). In the control group, cerebral FOE values did not significantly change (from 0.45 ± 0.08 to 0.46 ± 0.09, p = 0.377); however, the values in HD patients with severe anemia significantly decreased after HD with intradialytic blood transfusion (from 0.56 ± 0.10 to 0.52 ± 0.09, p = 0.002). Furthermore, cerebral FOE values in HD patients with severe anemia were significantly higher than those in the control group both before and after HD (p < 0.001 before and p = 0.043 after HD vs. controls).
Fig. 3.
a Comparison of hemoglobin levels before and after hemodialysis (HD) between the control group and HD patients with severe anemia. b Comparison of cerebral regional oxygen saturation (rSO2) values before and after HD between the control group and HD patients with severe anemia. * p < 0.001 versus before HD.
Table 3.
Comparison of cerebral fractional oxygen extraction before and after HD between the control group and HD patients with severe anemia
| Cerebral fractional oxygen extraction |
|||
|---|---|---|---|
| before HD | after HD | p value | |
| Controls (n = 25) | 0.45±0.08 | 0.46±0.09 | 0.377 |
| HD patients with severe anemia (n = 16) | 0.56±0.10 | 0.52±0.09 | 0.002 |
| p value | <0.001 | 0.043 | |
Values are presented as mean ± standard deviation. HD, hemodialysis.
Discussion
In this single-center observational study, we focused on the cerebral oxygenation of HD patients with severe anemia and confirmed the cerebral oxygenation change associated with intradialytic blood transfusion. The Hb levels in the included HD patients with severe anemia were extremely low compared to the target range recommended for renal anemia management, which is from 10 to 12 g/dL [15]. In general, Hb itself carries oxygen to the systemic tissue; therefore, the anemia could be a plausible cause of cerebral rSO2 deterioration via the systemic oxygen supply decrease, including that to the brain. Indeed, in this study, cerebral rSO2 values were significantly lower in HD patients with severe anemia than in those without severe anemia. Recently, Hb levels were reported to significantly correlate with cerebral oxygen saturation in a simple linear regression analysis in the field of pediatrics, intensive care medicine, and HD [6, 16, 17], and cerebral oxygen saturation was lower in patients with anemia than in those without anemia (with anemia: Hb levels 8.7 ± 2.3 g/dL, cerebral rSO2 50 ± 11%; without anemia: Hb levels 12.3 ± 4.2 g/dL, cerebral rSO2 66 ± 8%) [18]. Therefore, as expected, Hb levels would be one of the important factors associated with cerebral oxygenation in anemic patients with or without HD. In addition to the cerebral oxygen supply decrease induced by severe anemia, the significant decrease in serum albumin concentration in HD patients with severe anemia might be associated with the cerebral rSO2 reduction in this study. Colloid osmotic pressure is one of the important factors maintaining a systemic microcirculation (including that in the brain), and serum albumin concentration mainly constitutes the formation of colloid osmotic pressure [19]. In HD patients with well-maintained Hb levels, cerebral rSO2 was independently and positively associated with serum albumin concentration [6]. Therefore, a serum albumin decrease might lead to cerebral rSO2 reduction via a decrease in colloid osmotic pressure and cerebral microcirculation impairment.
Furthermore, several previous studies reported that blood transfusion improved cerebral oxygenation [10, 16, 18]; however, few investigated the relationship between intradialytic blood transfusion and cerebral oxygenation in HD patients. Thus, cerebral oxygenation improvement from intradialytic blood transfusion in this study was consistent with the results of those previous reports. Furthermore, even after intradialytic blood transfusion, Hb levels in the HD patients with severe anemia included in this study were significantly lower than in the control group (p < 0.001), and cerebral rSO2 levels in HD patients with severe anemia were also significantly lower than in the control group (p = 0.043). Therefore, in a clinical setting, we should make an effort to improve anemic conditions as soon as possible in HD patients with severe anemia. On the other hand, there were no changes in cerebral rSO2 in HD patients without intradialytic blood transfusion before and after HD. This might be explained by the absence of differences in Hb levels before and after HD; however, it was recently reported that cerebral oxygenation did not improve even with the significant increase in Hb levels induced by ultrafiltration during HD [7, 8]. Regarding the differences in changes in cerebral oxygenation between Hb increases by intradialytic blood transfusion and those by ultrafiltration during HD, the reasons might be explained by the changes in hematocrit-to-viscosity ratios [20] during HD. However, in this study, plasma viscosity was not measured; therefore, we cannot conclude an association between its ratio and changes in cerebral oxygenation.
In HD patients with severe anemia, cerebral oxygen supply decrease results in cerebral hypoxia with insufficient cerebral blood flow; therefore, cerebral FOE increases as a compensation to maintain cerebral oxygen metabolism even under severe anemia [16, 21]. The cerebral FOE values in the control group of a previous study were around 0.3 using the NIRS technology [9] and 0.4 using positron emission tomography [21]. In the present study, the cerebral FOE values in HD patients without intradialytic blood transfusion (control group) were relatively high compared to those in the previous reports [9, 21], and these values significantly increased further in those with severe anemia. Furthermore, cerebral FOE after HD significantly decreased with the Hb increase caused by intradialytic blood transfusion. It was previously reported that Hb levels negatively and significantly correlated with cerebral FOE values [9, 16] and significantly decreased to the normal range after blood transfusion [18]. In the present study, in addition to cerebral rSO2, cerebral FOE values remained high, at around 0.45–0.5, even in the control group and in HD patients after intradialytic blood transfusion; therefore, we should pay attention to the imbalance between oxygen delivery and consumption, particularly to oxygen delivery reduction, in all HD patients, even those with values within the target range of renal anemia management.
This study has several limitations. First, the sample size was small; thus, a large sample size is needed for the comparison of clinical parameters between severe anemic HD patients with intradialytic blood transfusion and controls, because the statistical power was relatively low. Second, cerebral oxygenation before the deterioration of Hb levels could not be measured, particularly in HD patients with severe anemia. Therefore, we cannot confirm the changes in cerebral oxygenation from well-maintained Hb levels to severe anemia. Furthermore, clinical parameters reflecting cerebral function could not be confirmed before and after intradialytic transfusion, so we cannot comment on the changes in these parameters throughout intradialytic blood transfusion in this study. Finally, HD therapy includes fluid and uremic substance removal; therefore, we cannot conclude that cerebral oxygenation improvement depended exclusively on intradialytic blood transfusion in this study. Further studies are needed to verify the associations between cerebral oxygenation and intradialytic blood transfusion in HD patients with severe anemia.
In conclusion, HD patients with severe anemia represented cerebral oxygen metabolism deterioration, which could be significantly improved by intradialytic blood transfusion.
Disclosure Statement
The authors declare that there is no conflict of interests in this work.
Acknowledgments
We thank the study participants and the clinical dialysis center staff in our hospital. This work was supported by a grant from the Japanese Association of Dialysis Physicians (No. 27088) to S. Ookawara, and a grant from The Kidney Foundation, Japan (JKFB16-3) to S. Ookawara.
References
- 1.Mix TC, Brenner RM, Cooper ME, de Zeeuw D, Ivanovich P, Levey AS, McGill JB, McMurray JJ, Parfrey PS, Parving HH, Pereira BJ, Remuzzi G, Singh AK, Solomon SD, Stehman-Breen C, Toto RD, Pfeffer MA. Rationale – Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT): evolving the management of cardiovascular risk in patients with chronic kidney disease. Am Heart J. 2005;149:408–413. doi: 10.1016/j.ahj.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 2.Galbusera M, Remuzzi G, Boccardo P. Treatment of bleeding in dialysis patients. Semin Dial. 2009;22:279–286. doi: 10.1111/j.1525-139X.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 3.Murkin MJ, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, Cleland A, Schaefer B, Irwin B, Fox S. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104:51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 4.Calderon-Arnulphi M, Alaraj A, Amin-Hanjani S, Mantukin WW, Polzonetti CM, Gratton E, Charbel FT. Detection of cerebral ischemia in neurovascular surgery using quantitative frequency-domain near-infrared spectroscopy. J Neurosurg. 2007;106:283–290. doi: 10.3171/jns.2007.106.2.283. [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama K, Ito N, Orita T, Hayashida K, Arimoto H, Beppu S, Unoki T, Endo T, Murai A, Hatada T, Yamada N, Mizobuchi M, Himeno H, Okuchi K, Yasuda H, Mochizuki T, Shiga K, Kikuchi M, Tsujimura Y, Hatanaka T, Nagao K, J-POP Registry Investigators Regional cerebral oxygen saturation monitoring for predicting interventional outcomes in patients following out-of-hospital cardiac arrest of presumed cardiac cause: a prospective, observational, multicentre study. Resuscitation. 2015;96:135–141. doi: 10.1016/j.resuscitation.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Ookawara S, Ueda Y, Goto S, Miyazawa H, Yamada H, Kitano T, Shindo M, Kaku Y, Hirai K, Yoshida M, Hoshino T, Nabata A, Mori H, Yoshida I, Kakei M, Tabei K. Factors affecting cerebral oxygenation in hemodialysis patients: cerebral oxygenation associates with pH, hemodialysis duration, serum albumin concentration, and diabetes mellitus. PLoS One. 2015;10:e0117474. doi: 10.1371/journal.pone.0117474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino T, Ookawara S, Goto S, Miyazawa H, Ito K, Ueda Y, Kaku Y, Hirai K, Nabata A, Mori H, Yoshida I, Tabei K. Evaluation of cerebral oxygenation in patients undergoing long-term hemodialysis. Nephron Clin Pract. 2014;126:57–61. doi: 10.1159/000358432. [DOI] [PubMed] [Google Scholar]
- 8.Malik J, Kudlicka J, Lachmanova J, Valerianova A, Rocinova K, Bartkova M, Tesar V. Tissue ischemia worsens during hemodialysis in end-stage renal disease patients. J Vasc Access. 2017;18:47–51. doi: 10.5301/jva.5000630. [DOI] [PubMed] [Google Scholar]
- 9.Wardle SW, Yoxall CW, Weindling AM. Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates. J Cereb Blood Flow Metab. 2000;20:272–279. doi: 10.1097/00004647-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Dani C, Pratesi S, Fontanelli G, Barp J, Bertini G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion. 2010;50:1220–1226. doi: 10.1111/j.1537-2995.2009.02575.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawanishi H, Akiba T, Masakane Y, Tomo T, Mineshima M, Kawasaki T, Hirakata H, Akizawa T. Standard on microbiological management of fluids for hemodialysis and related therapies by the Japanese Society for Dialysis Therapy 2008. Ther Apher Dial. 2009;13:161–166. doi: 10.1111/j.1744-9987.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 13.Tobias JD. Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Devices. 2006;3:235–243. doi: 10.1586/17434440.3.2.235. [DOI] [PubMed] [Google Scholar]
- 14.Lemmers PM, Toet MC, van Bel F. Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics. 2008;121:142–147. doi: 10.1542/peds.2007-0925. [DOI] [PubMed] [Google Scholar]
- 15.Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, Kuriyama S, Komatsu Y, Suzuki M, Nakai S, Hattori M, Babazono T, Hiramatsu M, Yamamoto H, Bessho M, Akizawa T. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial. 2010;14:240–275. doi: 10.1111/j.1744-9987.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 16.van Hoften JCR, Verhagen EA, Keating PK, ter Horst HJ, Bos AF. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch Dis Child Fetal Neonatal Ed. 2010;95:F352–F358. doi: 10.1136/adc.2009.163592. [DOI] [PubMed] [Google Scholar]
- 17.Ameloot K, Gnebrugge C, Meex I, Janssens S, Boer W, Mullens W, Ferdinande B, Dupont M, Dens J, De Deyne C. Low hemoglobin levels are associated with lower cerebral saturations and poor outcome after cardiac arrest. Resuscitation. 2015;96:280–286. doi: 10.1016/j.resuscitation.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Sandal G, Oguz SS, Erdeve O, Akar M, Uras N, Dilmen U. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion. 2014;54:1100–1105. doi: 10.1111/trf.12359. [DOI] [PubMed] [Google Scholar]
- 19.Ookawara S, Sato H, Takeda H, Tabei K. Method for approximating colloid osmotic pressure in long-term hemodialysis patients. Ther Apher Dial. 2014;18:202–207. doi: 10.1111/1744-9987.12070. [DOI] [PubMed] [Google Scholar]
- 20.Waltz X, Hardy-Dessources MD, Lemonne N, Mougenel D, Lalanne-Mistrih ML, Lamarre Y, Tarer V, Tressieres B, Etienne-Julan M, Hue O, Connes P. Is there a relationship between the hematocrit-to-viscosity ratio and microvascular oxygenation in brain and muscle? Clin Hemorheol Microcirc. 2015;59:37–43. doi: 10.3233/CH-131742. [DOI] [PubMed] [Google Scholar]
- 21.Kuwabata Y, Sasaki M, Hirakata H, Koga H, Nakagawa M, Chen T, Kaneko K, Masuda K, Fujishima M. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int. 2002;61:564–569. doi: 10.1046/j.1523-1755.2002.00142.x. [DOI] [PubMed] [Google Scholar]